-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2025; 15(1): 7-16
doi:10.5923/j.microbiology.20251501.02
Received: Sep. 17, 2025; Accepted: Oct. 8, 2025; Published: Oct. 25, 2025

Comparative Evaluation of Antimicrobial Activities of Biogenic Silver Nanoparticles Synthesized from Tropical Fruit Components
Bright Ankudze
University of Education, Winneba, Ghana
Correspondence to: Bright Ankudze, University of Education, Winneba, Ghana.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Extracts from plants are known to possess crucial phytochemicals essential for reduction and stabilization of nanoparticles. Phytochemicals can increase the potency of the nanoparticles and widen their scope of application. Studies have shown that nanoparticles behave differently when prepared with different plants extracts. Hence, comparative study of different biogenically prepared nanoparticles can increase understanding of the effect of surface functionalization in selective antimicrobial activity and potency of nanoparticles. In a previous studies, different silver nanoparticles were prepared using Chrysophyllum albidum peel (Alb-AgNPs), Cyperus esculentus tuber (CpE-AgNPs), and Dialium cochinchinense (VeV-AgNPs) husk. In the present study, a comprehensive comparison is made among the prepared silver nanoparticles to ascertain their antimicrobial efficacy. The result showed that VeV-AgNPs had the strongest activity, with minimum inhibitory concentrations as low as 3.9 µg/mL against E. coli, and synergistic effects with ciprofloxacin. Alb-AgNPs exhibited broad-spectrum efficacy, notably against E. coli and C. albicans, and enhanced antibiotic activity, whereas CpE-AgNPs demonstrated moderate effects but strong synergy at low doses and broader spectrum against wider range of microorganism. In antibiofilm assays, CpE-AgNPs were highly effective against S. mutans and MRSA at low concentrations, Alb-AgNPs showed consistent but dose-dependent inhibition, and VeV-AgNPs showed ability to significantly disrupt preformed biofilms across bacterial and fungal strains.
Keywords: Silver nanoparticles, Green synthesis, Phytochemicals, Antimicrobial activity, Biofilm inhibition, Synergistic effect
Cite this paper: Bright Ankudze, Comparative Evaluation of Antimicrobial Activities of Biogenic Silver Nanoparticles Synthesized from Tropical Fruit Components, Journal of Microbiology Research, Vol. 15 No. 1, 2025, pp. 7-16. doi: 10.5923/j.microbiology.20251501.02.
Article Outline
1. Introduction
- The advancement of medicine has led to numerous breakthroughs in the fight against difficult-to-treat and emerging diseases through the development of highly potent antiviral and antimicrobial agents [1-7]. Over the years, nanotechnology has emerged as a transformative field with wide-ranging applications in medicine [8], agriculture, food preservation, and environmental remediation [9,10]. Among the various nanomaterials, silver nanoparticles (AgNPs) have received particular attention owing to their remarkable antimicrobial [11], antioxidant [12], and catalytic properties [13]. The synthesis of AgNPs can be achieved through physical, chemical, or biological approaches. Physical methods such as laser ablation and evaporation-condensation [14], and chemical reduction using stabilizing agents [15], allow fine control over particle size and morphology, however, they are often limited by high energy demands and the generation of toxic by-products. Biological or green synthesis, on the other hand, offers a sustainable and eco-friendly alternative, relying on natural biomolecules to drive nanoparticle formation [16].Green synthesis leverages the reducing and stabilizing agents present in plant [17], algal [18], fungal [19], and animal-derived extracts, which replace harsh chemical reagents with biogenic molecules. Plant-derived extracts, in particular, are rich in phenolics, flavonoids, terpenoids, alkaloids, and polysaccharides, all of which facilitate both the reduction of metal ions and the stabilization of the resulting nanoparticles [20]. Leaves and fruit peels from species such as Azadirachta indica (neem), Medicago sativa [21], Coriandrum sativum [22], rice [23], and cabbage [24], have been widely used for the biosynthesis of nanoparticles, producing stable nanostructures under mild conditions. Beyond plants, algal and fungal extracts provide enzymatic pathways that influence particle size and morphology, while animal-derived materials such as proteins (egg white, milk casein, gelatin, collagen, silk fibroin), polysaccharides (chitosan), and natural products like honey act as templates or stabilizers, yielding biocompatible nanoparticles with diverse applications [25].Biologically mediated nanostructures are increasingly being explored across multiple sectors. In environmental remediation, they play roles in dye degradation, pollutant removal, and photocatalysis [25]. In agriculture, they serve as nano-fertilizers, seed priming agents, and nano-pesticides that enhance nutrient use efficiency and plant resilience [25]. In medicine, their potential as antimicrobial coatings, wound dressings, targeted drug delivery systems [26,27], and cancer theranostics has gained significant momentum. The versatility of these applications underscores the importance of synthesis methods and precursor biomaterials in determining the physicochemical properties and functional performance of AgNPs.Despite the versatility and wider application of green synthesized nanoparticles, growing concerns about the climate crisis have highlighted the need for more sustainable and environmentally friendly approaches in green synthesis of silver nanoparticles [28]. Significant strides have been made to make nanomaterial synthesis greener, with particular attention to replacing heavily dependent biomaterials with less-used or discarded bioresources. The use of discarded plant waste as precursor materials for nanoparticle synthesis provides an added layer of significance [29]. They do not only help reduce environmental pollution but also offers a low-cost, eco-friendly pathway for producing valuable nanomaterials. As a result, much effort has gone into advocating the use of discarded plant wastes such as fruit peels, seed shells, vegetable residues [30] and agro-biomass [23] for the green synthesis of silver nanoparticles. Among promising discarded wastes, peels of Chrysophyllum albidum and husks of Dialium cochinchinense have a rich phytochemical composition and widespread availability. Peels of Chrysophyllum albidium have nutritional and therapeutic properties, and can be used for green synthesis of AgNPs, yielding nanoparticles with strong antibacterial and antibiofilm activity. Husk of Dialium cochinchinense [31–33] traditionally consumed for its sweet-sour pulp and used in ethnomedicine, has also been successfully applied in nanoparticle synthesis using discarded fruit husks under sunlight exposure, producing effective antimicrobial agents. Another widely available plant resource, especially in the tropical regions that have been explored for biogenic synthesis of silver nanoparticles is tiger nuts [34]. Tiger nut (Cyperus esculentus) tubers are nutritionally dense, containing oils, starch, fibre, and polyphenolics, which have been linked to health benefits and, more recently, have been exploited for AgNP synthesis with notable antimicrobial outcomes [35]. As different biomaterials provide unique phytochemicals that act as reducing and capping agents, comparative studies that evaluate nanoparticles synthesized from diverse biomaterials are therefore essential to identify the most effective synthesis pathways and selective performance for targeted application. Such studies are particularly relevant in the current era, where antimicrobial resistance poses a pressing global health challenge. In previous studies, silver nanoparticles were synthesized using different extracts from Cyperus esculentus [35] tubers, Chrysophyllum albidum [36] peels, and Dialium cochinchinense [37] husks as reducing and stabilizing agents. The obtained silver nanoparticles were studied for their antimicrobial properties. In the present study, a comparative evaluation of nanoparticles derived from Cyperus esculentus, Chrysophyllum albidum, and Dialium cochinchinense was presented. This comparative study is crucial to determine their relative antimicrobial performance and identify the most promising candidates for practical application.
2. Experimental
2.1. Chemicals
- Silver nitrate (AgNO3, Merck, ≥ 99 %) was used as a precursor for the synthesis of silver nanoparticles. Chrysophyllum albidum, Cyperus esculentus, Dialium cochinchinense fruits were used as reducing agents in separate synthesis to prepare different sets of silver nanoparticles. The fruits were purchased from the local market. Hypochlorite solution was used to disinfect the Chrysophyllum albidum fruit peel, Dialium cochinchinense, and Cyperus esculentus fruit before use. Methanol (Sigma Aldrich, analytical grade), Phosphate buffer saline (PBS), Mueller-Hinton Broth (Oxoid, USA), MTT (3-(4,5- dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide, 0.1%, w/v, Sigma Aldrich), Phosphate Buffered Saline (PBS, Sigma Aldrich, analytical grade), McFarland standard (barium chloride and sulphuric acid) were used to study the antimicrobial properties of the prepared silver nanoparticles.
2.2. Synthesis of AgNPs
- Three different silver nanoparticles were prepared, namely: CpE-AgNPs, Alb-AgNPs and VeV-AgNPs using Cyperus esculentus, Chrysophyllum albidum and Dialium cochinchinense as bio-reducing and stabilizing agents. Each bio material was used as a reducing agent to prepare one type of silver nanoparticles. In all, three different types of silver nanoparticle were prepared using the different bio-reducing agents. First extracts were prepared by soaking 7g, 1g and 2.5g of Cyperus esculentus tuber, Chrysophyllum albidum dried peels and Dialium cochinchinense husk, respectively, in 40 mL of water for 24 hours. To prepare the AgNPs, 40 mL of extracts were mixed with 2 mL of 0.01 M AgNO3, stirred, and exposed to sunlight. The formation of the AgNPs were observed a color change from colourless, pale-yellow and pale yellow, to dark red for extract obtained from Cyperus esculentus tuber, Chrysophyllum albidum dried peels and Dialium cochinchinense husk, respectively. After centrifugation, the particles were separated and stored for further analysis.
2.3. Characterization of Prepared AgNPs
- The synthesized AgNPs were characterized using: UV-Vis spectrophotometer to confirm plasmonic absorption. Scanning electron microscopy (SEM) for morphological analysis. X-ray diffraction (XRD) to determine crystallinity. Fourier-transform infrared spectroscopy (FTIR) to identify functional groups involved in stabilization.
2.4. Test Organisms
- The microorganisms used as test organisms for the study include Methicillin-Resistant Staphylococcus aureus (NCTC 29212), Candida albicans (ATCC 90028), Aspegillus niger (ATCC 6275), Klebsiella pneumoniae (NCTC 13440), Salmonella typhi (ATCC14028), Streptococcus mutans (ATCC 700610), Pseudomonas aeruginosa (ATCC 4853) and Escherichia coli (ATCC25922).
2.5. Determination of Antimicrobial Activity
- To evaluate the antimicrobial properties of CpE-AgNPs, Alb-AgNPs and VeV-AgNPs, the broth micro-dilution method was employed using a 96 well microtiter plates according to previously established procedure (Clinical and Laboratory Standard Institute, [38]. For this study, 1 mg/mL AgNPs were prepared from each of the different silver nanoparticles. The preparation involved dispensing 100 μL of double-strength Mueller-Hinton broth into each well of a 96-well plate, which was then mixed with 100 μL AgNPs to create well concentrations ranging from 250.0 –0.1 mg/mL using the stock solution, resulting in ten different concentrations. For each column in the microtiter plate, wells 11 and 12 were utilized as the positive control (Broth + organism only) and negative control (Broth with no organism), respectively, for each microbial strain. Similarly, separate plates were prepared for antibiotics, namely ciprofloxacin, tetracycline, ampicillin, ketoconazole, fluconazole, and nystatin, and their concentrations were varied from 128.0 – 0.125 µg/mL, against all test bacteria and fungi. Subsequently, 100 µL of each of the 0.5 McFarland standard was added to the test organisms and the plates were then incubated at 37°C for 24-48 hours for bacterial and fungal strains, respectively. The minimum inhibitory concentrations (MICs) were determined by visual examination following the addition of tetrazolium chloride (TTC) dye at a concentration of 0.1 g/mL after 10 minutes. The MICs were determined as the lowest concentration that did not change color from colorless/light yellow to red/pink.
2.6. Minimum Bactericidal (MBC) and Fungicidal Concentration (MFC) Determination
- To investigate the microbial cell-killing potential (bactericidal/fungicidal effect) of the AgNPs, the minimum bactericidal/fungicidal concentrations (MBC/MFC) were determined. For this, samples from each well of the susceptibility testing assays were transferred onto sterile nutrient agar plates and incubated for 24/48 hours at 37°C for bacteria/fungi, respectively. After incubation, the plates were examined for the presence or absence of growth on the nutrient agar (Nester et al. 2004).
2.7. Evaluation of Synergistic Effects of the Test AgNPs and Antibiotics
- The combinatory effects of the AgNPs and antimicrobial orthodox drugs were studied against the test microbes using the checkerboard method, with minor modifications based on previous studies by Khodavandi et al. (2010) and Nascimento Da Silva et al. (2013). In brief, solutions with various proportions of AgNPs and drugs (final volume of 200 μL) were prepared from twice the MIC solutions of each test sample and individual antibiotics (1 mg/mL). The antimicrobial activity of these solutions was determined, as described for MIC determination. The Fractional Inhibitory Concentration index (FICI) was calculated using equation (1).
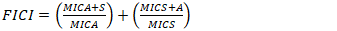 | (1) |
2.8. Determination of Anti-Biofilm Formation Activity of the Prepared AgNPs
- The antibiofilm activity of the AgNPs against the selected microorganisms was studied using the protocol by Pierce et al. (2008) with little modification. In brief, about 50 µL of MHB was added to each well of a flat 96-well microtiter plate. The different AgNPs samples (50 µL) were serially diluted to obtain concentration within the range of 500 to 0.197 µg/mL. Afterwards, the microbial suspensions (50 µL, 2 × 106 cells/mL) were added to wells of columns 1-11, and the plates were incubated for 24 h at 37°C. After the incubation, the liquid was removed with a pipette without disturbing the biofilm. PBS (100 µL) was used twice to wash away and remove any planktonic and non-adherent cells. The postprocessing to quantify the metabolic activity after the antimicrobial treatment were checked by XTT reduction assay as previously described by Pierce et al. (2008) with slight modifications. The microtiter plates were then read at 490 nm using a spectrophotometer. The procedure was repeated three times. The potential of each of the AgNPs samples to reduce the optical density compared to the negative control was noted as the biofilm inhibitory activity as described by equation (2).
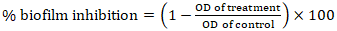 | (2) |
3. Results and Discussion
3.1. Structural Studies of Alb-AgNPs, VeV-AgNPs and CpE-AgNPs
- The morphology of the prepared silver nanoparticles was observed using scanning electron microscope. It can be observed in Figure 1 that Alb-AgNPs were quasi-spherical but polydisperse, with a broader size range (28–90 nm) and particles occasionally exceeding 100 nm, suggesting less uniformity. CpE-AgNPs, on the other hand, were mostly spherical to quasi-spherical within the range of 50–80 nm, with occasional rods. VeV-AgNPs were predominantly quasi-spherical with a mean size of 45.0 ± 8.5 nm, though occasional rodlike shapes were observed. As presented in Table 1, while all three extracts facilitated nanoparticle formation, Dialium cochinchinense extract appeared to produce smaller and more uniform particles compared to the other two.
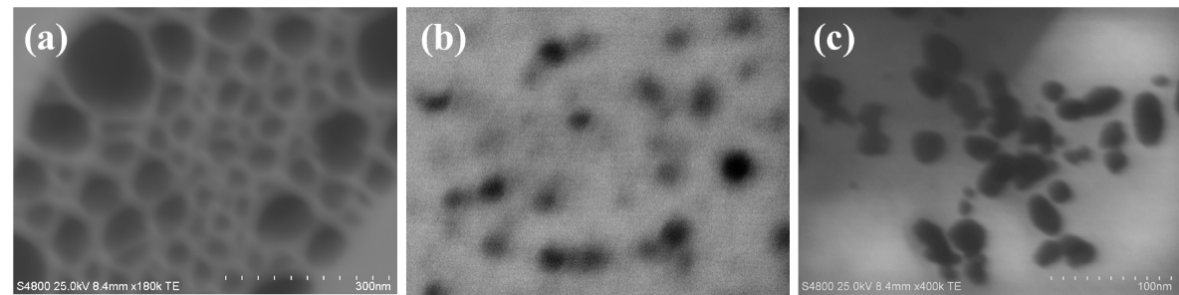 | Figure 1. STEM images of (a) Alb-AgNPs (b) CpE-AgNPs, (c) VeV-AgNPs |
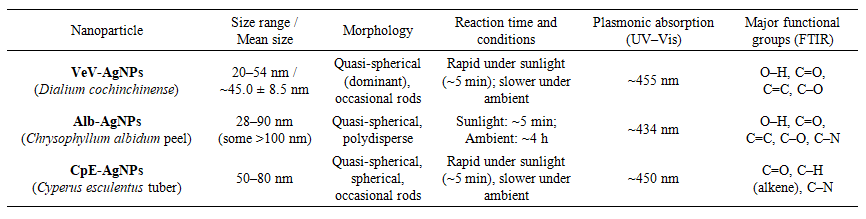 | Table 1. Structural properties of VeV-AgNPs, Alb-AgNPs and CpE-AgNPs |
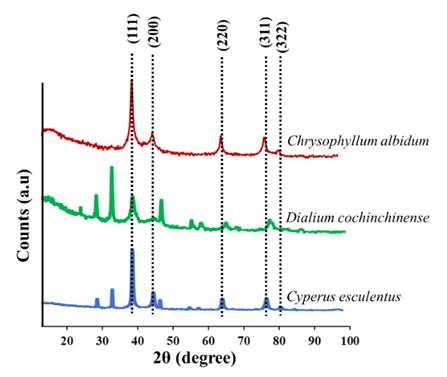 | Figure 2. P-XRD pattern of prepared silver nanoparticles showing the various diffraction planes |
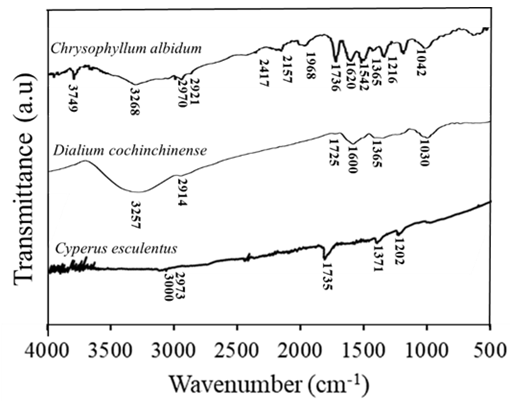 | Figure 3. FTIR spectrum of the prepared silver nanoparticles |
3.2. Antibacterial Analyses of VeV-AgNPs, Alb-AgNPs and CpE-AgNPs
- Alb-AgNPs exhibited significant antimicrobial effects across a broad range of bacterial and fungal pathogens. They demonstrated strong activity against E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis, S. mutans, and C. albicans, with minimum inhibitory concentration (MIC) values as low as 15.62 µg/mL for some strains. Notably, Alb-AgNPs outperformed silver nitrate (AgNO₃) in several cases, particularly against E. coli and C. albicans. Synergistic studies, as presented in Table 2, showed that Alb-AgNPs enhanced the activity of tetracycline against K. pneumoniae, S. mutans, and B. subtilis, as well as ciprofloxacin against MRSA and P. aeruginosa. This suggests that Alb-AgNPs are effective both as standalone antimicrobials and as enhancers of antibiotic action.
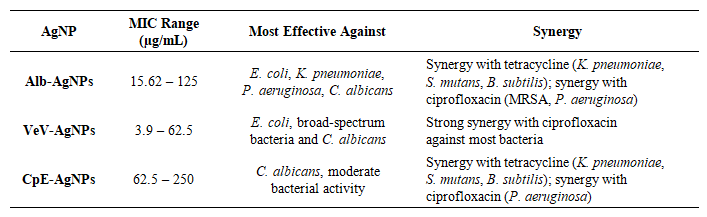 | Table 2. Antimicrobial studies (MIC and synergistic properties) of the prepared silver nanoparticles |
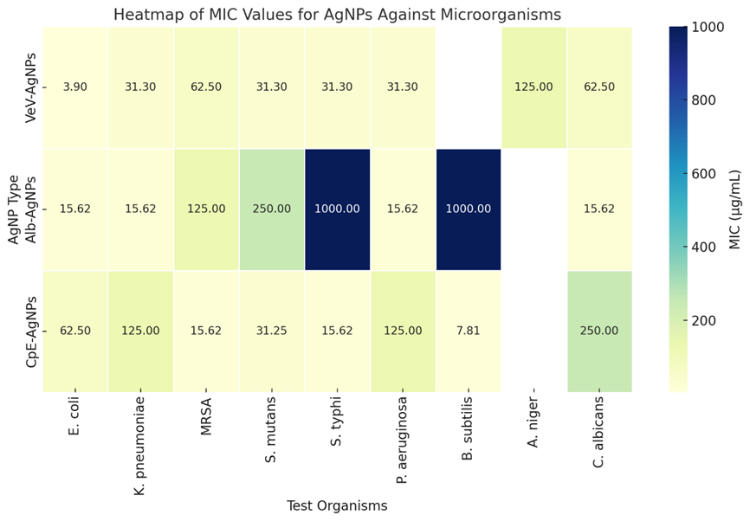 | Figure 4. Heatmap showing the sensitivity of the prepared silver nanoparticles against tested microorganisms. Darker colours represent lower MICs |
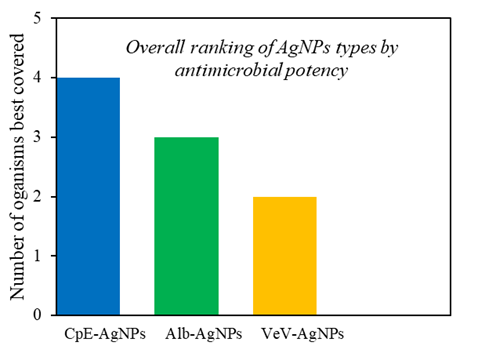 | Figure 5. Ranking of prepared silver nanoparticles in terms of coverage |
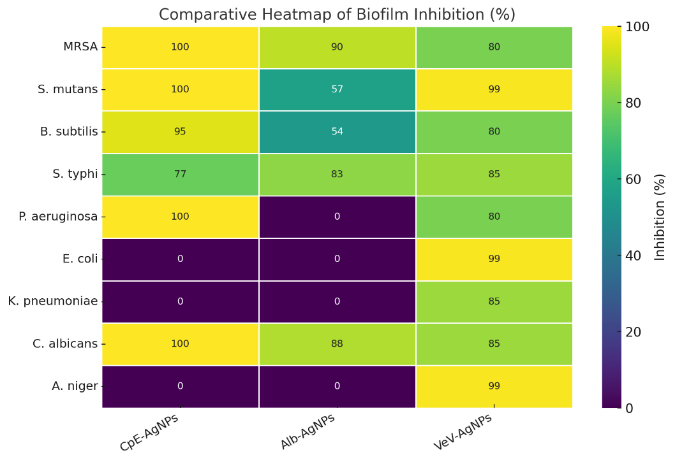
3.3. Antibiofilm Properties of the Prepared Silver Nanoparticles
- The CpE-AgNPs exhibited strong antibiofilm activity against a broad spectrum of microbes, including S. mutans, MRSA, B. subtilis, P. aeruginosa, S. typhi, and C. Albicans (Table 3). Remarkably, complete inhibition (100%) of MRSA and S. mutans biofilms was achieved at relatively low concentrations (125 µg/mL and 62.5 µg/mL, respectively), far lower than what was required by previously reported AgNPs. Against P. aeruginosa and C. albicans, 100% inhibition occurred only at higher concentrations (250 µg/mL), but even low doses yielded significant activity (>50% inhibition at <20 µg/mL). The order of sensitivity at 62.5 µg/mL was S. mutans > MRSA > C. albicans > P. aeruginosa > S. typhi > B. subtilis. This shows CpE-AgNPs are particularly potent against oral pathogens (S. mutans) and resistant bacteria (MRSA). Alb-AgNPs also displayed broad antibiofilm activity but were generally less potent than CpE-AgNPs. At the maximum tested concentration (250 µg/mL), inhibition ranged from ~54% (B. subtilis) to > 90% (MRSA). Against S. typhi and C. albicans, the inhibition was high (83% and 88% respectively), while for S. mutans it was more moderate (57%). Compared to CpE-AgNPs, Alb-AgNPs required higher concentrations to achieve significant inhibition, but their performance against C. albicans was notable. The sensitivity trend was MRSA > S. typhi > C. albicans > S. mutans > B. subtilis.
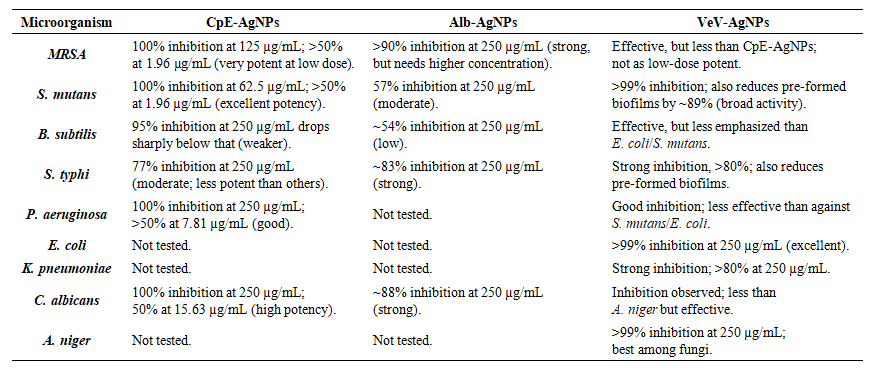 | Table 3. Antibiofilm performance of the prepared silver nanoparticles |
4. Conclusions
- In this work, different silver nanoparticles, CpE-AgNPs, Alb-AgNPs and VeV-AgNPs biogenically prepared were compared to study their antimicrobial properties. This is important to rationalized the effect of surface functionalization on antimicrobial properties. In terms of effectiveness, VeV-AgNPs showed the most versatility, exhibiting ability to inhibit microbial growth even at a very low concentration. CpE-AgNPs, though with moderate antimicrobial action showed a broader antimicrobial spectrum against wider range of microorganisms highlighting their potential in therapeutic applications. Alb-AgNPs displayed reliable broad-spectrum activity with notable synergistic effects alongside conventional antibiotics. Collectively, these findings emphasize the role of phytochemical composition in shaping nanoparticle performance and support the development of green nanotechnology as a sustainable approach for addressing microbial resistance.
ACKNOWLEDGEMENTS
- The authors acknowledge the contributions of Christopher Dawari and the University of Eastern Finland for SEM imaging.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML