-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2022; 12(1): 24-33
doi:10.5923/j.microbiology.20221201.03
Received: Feb. 25, 2022; Accepted: Mar. 11, 2022; Published: Mar. 24, 2022

Viral Respiratory Co-Infections in Patients in the Intensive Care Unit and Those under Mechanical Ventilation in Moi Teaching and Referral Hospital, Eldoret; Kenya
Kipsang A. K., Arodi W. O.
Department of Medical Laboratory Science, Kenyatta University, Kenya
Correspondence to: Kipsang A. K., Department of Medical Laboratory Science, Kenyatta University, Kenya.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Ventilator support as a component of critical care is rarely utilized. It only supports the breathing system but does not change any pre-existing condition. Associated challenges include viral and bacterial infections. The prevalence of these infections is high, however most healthcare workers confuse them as bacterial infections. They impact emotional, economic and psycho-social burden on individuals, families as well as the society at large. There is no documentation of these infections in many healthcare facilities. The study aimed at assessing the prevalence rates of Influenza virus, HRV, RSV, HPIV, hMPV, Human Adenovirus and HCOV in study subjects on ventilator support as well as on critical care in the ICU.The study was done at MTRH in Uasin Gishu County. Samples were collected from January 2017 to December 2017. 200 samples of bronchoalveolar lavage were collected. The samples were then transported to KEMRI Nairobi at 2-8°C for analysis. The RNA/DNA of the viruses was detected using real time PCR. Data analysis as well as coding and entry were done using statistical package for social studies (SPSS). The results were log-transformed to obtain equal distribution. The results were also expressed as mean±standard deviation. The results were then compared with respect to whether in ICU or on mechanical ventilation as well age and gender using ANOVA with Bonferroni’s post-test using GenStat Release 14.1 (PC/Windows). Presentation of the data was done using graphs, pie charts and tables/figures. 34 cases of multiple viral infections was identified, 20 cases in MV and 14 cases in ICU. Two viruses were detected in 22 samples and three viruses in 12 samples. There was high prevalence of co-infections by Influenza A virus/PIV-3/hMPV at 4% and 3% in the ICU and those on ventilator support respectively. It is evident that these infections are common in patients in ICU and those under ventilator support at MTRH. Surveillance for viral respiratory infections should be improved in order to implement treatment and also understand seasonality of these viruses and other new respiratory viruses.
Keywords: Prevalence, Respiratory, Ventilation, Polymerase, Co-infection
Cite this paper: Kipsang A. K., Arodi W. O., Viral Respiratory Co-Infections in Patients in the Intensive Care Unit and Those under Mechanical Ventilation in Moi Teaching and Referral Hospital, Eldoret; Kenya, Journal of Microbiology Research, Vol. 12 No. 1, 2022, pp. 24-33. doi: 10.5923/j.microbiology.20221201.03.
Article Outline
1. Introduction
- There is close association between mechanical ventilation and pneumonia due to mechanical ventilation as well as other associated risks like airway injury. This can lead to alveolar damage and pneumothorax. It can also cause decreased cardiac output, oxygen toxicity diaphragm atrophy. Serious complications that may occur in patients on ventilator support include acute respiratory distress syndrome (ARDS) and acute lung injury [1].Patients in the ICU are closely monitored and are usually kept comfortable by the medication that they are given. However consciousness in these patients may diminish, but sedation level may be different from patient to patient, and also condition of the patient. They become easily aroused and are also able to converse. Other patients may need higher levels of sedation to an extent of not being responsive to stimulation [2].One of the most common reasons for people being admitted into intensive care is severe pneumonia. Some people have symptoms that are similar to common cold or flu. When their health deteriorates and they have difficulties breathing, they get admitted in hospital and later into intensive care. Some people who have not been admitted because of pneumonia have septicemia. This is a serious infection which affects the circulation as well as the lungs [3].These infections are common in humans regardless of their age or gender. They cause higher levels of morbidity at community level. The prevalence annually of theses illnesses increase in children at 2years and fall during the coming years. These infections will then increase at child bearing age then decreases as an individual grows older. However infections tend to increase in the elderly population [4].Infections of the respiratory tract by viruses are common in all individuals regardless of their age. Adults and children are mostly affected. It also increases mortality in older adults and those with chronic diseases. For instance, 90% of seasonal influenza and 78% of RSV infections occur in adults aged above 65 years in the US, however many studies on RSV, Human Metapneumovirus virus and HRV focus on children below 5 years and elderly [5].Antiviral treatment of these viruses is available for Influenza Virus. Immediate diagnosis of these infections by viruses is important in management of patients admitted in the hospital, thus accurate and prompt diagnosis of these infections will help understand the cause of disease and reduce spread of the infection. These infections should be diagnosed early to help to stop administration of unnecessary antimicrobial agents and begin the use of antiviral drugs like for influenza. This will in turn reduce cost by shortening length of hospital stay [6].
2. Literature Review
- Overview of mechanical ventilation.Mechanical ventilation is an important aspect of intensive care however technical issues make it complicated and difficult for majority of the healthcare providers. The recent trend in this area of respiratory medicine worsens the situation. Most of the available literature on complications of ventilator support applies to a few patients who are on intubation and ventilator support [7] This small proportion includes those with respiratory failure due to ALI or ARDS. This also applies to those with chronic pulmonary disease (COPD) or asthma. Less difficult issues occur in the remaining 80 to 90 percent of ventilated patients [8].Mechanical ventilation supports gas exchange and normal functioning of the lungs. It is therefore a form of replacement therapy since it replaces functioning of the lungs in a normal fashion however it doesn’t cure any underlying disease condition. Ventilator support may replace normal functioning of the lungs and the chest if it is done properly, however due to the adverse effects of ventilator support and positive pressure associated ventilation as well as intubation, it is advisable to reduce the time of ventilation as much as possible [9].Overview of viral respiratory tract infections.Viral infections contribute to higher respiratory infections than previously thought; approximately 5% of these infections are due to viruses, but this has been underestimated [10] The risk of infection by these viruses tends to increase when there is an epidemic. The impact of nosocomial viral respiratory infections has been neglected for a long time especially due to the challenges encountered in diagnosis and non-specific clinical manifestations of these infections [11].Viral infections are common in infants born prematurely and young children; however there is increased risk of infection by these viruses in preterm infants, the immunosuppressed and those with pulmonary diseases. In addition reinfection is more frequent in these groups of patients [12] Neonatal infections are not well understood and there is little literature available describing these infections. Early diagnosis will help the clinicians to choose correct therapeutic measures and control the spread of viral pathogens within the healthcare facility [13].In children below 5 years, mortality due to respiratory infections is estimated at 20% especially in underdeveloped countries, (Scott et al., 2008). The cause of pneumonia in the community includes Human Rhinovirus, Respiratory Syncytial virus and Bocavirus. According to WHO report (WHO., 2008), respiratory viral infections account for greater than 90% of viral bronchiolitis cases in infants, an estimated 50% of pneumonia in the community in young children and over 90% of worsening asthma cases in children. 30-50% of CAP and 20-60% in cases of chronic lung disease in the elderly people have been associated to these infections.Viruses that infect the respiratory tract.Adenovirus.Adenovirus is a Ds DNA virus. It is not enveloped, however; it causes illness in humans but illnesses are not severe. Often it causes respiratory illness but may also cause fever, diarrhea, conjunctivitis, bladder infection and a rash. Anyone can get infected especially infants and people with weakened immune response or an existing respiratory disease like tuberculosis. The incubation period is 2-6 days [14,15].Human RhinovirusHuman Rhinoviruses is +sense, ssRNA virus. It is not enveloped, belonging to Picornaviridae family. They are associated with common cold but may also cause ear infection, sore throat, pneumonia and bronchitis. The incubation period is 8 hours to 2 days [16,4].Influenza virusInfluenza virus is enveloped with a segmented genome. The genome is –ssRNA. It is an Orthomyxovirus. It is the causative agent of flu. This virus has three genera, A, B, and C. Influenza A causes severe disease [17] The virus is spread through inhalation of aerosols produced by an infected person. It can also be transmitted through contact to the eye or hand to nose and also hand to mouth transmission. Symptoms appear after 1-2 days [18].Parainfluenza VirusParainfluenza Viruses (PIV) is ssRNA virus. It is in the family of Paramyxoviridae family. In young children they cause pneumonia and bronchitis [19] Endemic infections are mainly due to PIV type 3, while infections caused by PIV-1 and PIV-2 rise as the months fall [20] Reinfection occurs with advancing age. Acute respiratory illnesses due to this virus account for 1%–15% of respiratory infections in young adults. Symptoms appear after 2-8 days [12].Respiratory Syncytial VirusRSV is an RNA virus in the family of Paramyxoviridae [21] It causes a large proportion of LRTI in infancy and childhood. It is an agent of bronchiolitis and pneumonia in young children and infants [22] This has contributed in increased burden on health services. Vaccine development for RSV is a major concern worldwide. Information is accessible on the epidemiological features and clinical presentation of this virus especially in young patients with infections of the respiratory tract in hospitals [23] In the elderly population no much is known about infections by RSV [24].Human CoronavirusCoronaviruses are RNA viruses with crown-like appearance. They were discovered in 1965. It causes common cold just like rhinovirus [25] Growing this virus in cell culture has been difficult. This has led to limited studies over the past years [26,27].Human MetapneumovirushMPV is an RNA virus. It belongs to the family of Paramyxoviridae [28] It causes acute viral respiratory tract infection in children. It also affects the immuno-suppressed and the elderly. Global prevalence of this virus is approximately 5-15%. In developing countries especially Africa, data is not available on genetic diversity and epidemiology of this virus [29].Epidemiology of viral respiratory virusesViral respiratory tract infections cause a huge burden on individuals, families and society. These infections cause illnesses in children and also the elderly people worldwide. URTI are prevalent in young children and infants. These infections will continue to be more prevalent in adults and older children. 3–8 episodes of cold annually occur in young children and infants. Those attending daycare centers usually have more episodes per year [30] Diagnosis of viruses such as RSV, Influenza Viruses and Adenoviruses was easily done using traditional methods but as time passed by other viruses like HCOV and HRV were discovered. Detection methods have also improved. 50% and 75% of upper respiratory tract infections are attributed to HCOV and HRV [31].Human Rhinovirus has recorded a higher prevalence in most regions of Kenya. A prevalence of (33%) has been recorded along the coastal region, the Western and eastern region has a prevalence of 32.7% and 11% respectively. However those aged between 2 months -7 years have highest infection rates. Those above 60 years had low infection rates [32].There is insufficient data on Influenza Virus in sub-Saharan Africa because of poor disease surveillance. This is a setback in detection of other strains of Influenza Virus that could have an effect on influenza pandemic [33] Kenya has a good framework for surveillance of Influenza virus countrywide. However it is a main cause of hospitalizations and deaths annually in Kenya [34].In Kenya, Influenza Virus is common all through the year but with some peaks at some seasons of the year. The incidence is high during the winter and also during the rainy months: March-April, October-November and cold month of July [35].The patterns of epidemiology and evolution of circulating strains of hMPV are not well documented sub-Saharan Africa. From 2000 to 2011 4.8% of hospitalizations due to pneumonia in children were positive for hMPV in Kilifi [36].Human Coronavirus are endemic to many countries globally [37,38] There is little information about coronavirus especially in sub-Saharan Africa. The diversity, molecular features and circulation dynamics is not well documented [39].The incidence of respiratory disease in infants in urban and rural settings associated with RSV is high in Kenya (Godfrey et al., 2013). 470 (12%) were positive for RSV among 4714 children hospitalized with acute respiratory infection [40].
3. Materials and Methods
3.1. Study Area
- This study was done at MTRH, Uasin Gishu County. This hospital is in the town of Eldoret; county of Uasin Gishu located in the Rift Valley. This county is neighbouring to Trans Nzoia County, Elgeyo marakwet County, Baringo County, Kericho County and Nandi County [41].
3.1.1. Population
- The population of Uasin Gishu was 894,179 according to housing and population census of 2009. The growth rate of the population was 3.8% while the density of the population was 267 persons per square kilometer.
3.1.2. Climate
- This county experiences even distribution of high and reliable rainfall. The average rainfall is between 620mm to 1,560mm. April and May are the wettest months while January and February are dry months. The annual temperature is between 7°C and 29°C.
3.1.3. Catchment Area
- The bed capacity at MTRH is 800. The hospital serves a large population in the neighbouring counties of West Pokot, Nandi, Turkana, ElgeyoMarakwet, Bungoma, and Tranzoia. It also receives patients from parts of Sudan and Uganda.
3.2. Study Population
- It comprised of consented study participants in the ICU and those on ventilator support in MTRH.
3.3. Study Design
- The design of this study was cross-sectional study. Samples collected from consented participants were tested for Influenza virus, PIV, HRV ADV, RSV, HCOV and hMPV using Real time polymerase chain reaction.
3.4. Sampling Technique
- All patients under mechanical ventilation and those in the ICU who consented were sampled.
3.5. Sample Size Determination
- The sample size calculation was determined by the formula of Fisher et al., 1998 based on 95% level of confidence and anticipated prevalence of 85.9%. The estimated prevalence was based on an earlier study in Thailand [42]N=Z2 X P (I-P)/E2Where:N = Desired sample sizeZ = Standard normal deviation = 1.96 (from the tailed normal table)P = Incidence rate of viral respiratory infectionsE = the desired degree of accuracy at 95% confidence level = 0.5N = 1.962 X 0.859(0.141)/0.05=186 samples.A total of 186 study participants in the ICU and those on ventilator support at MTRH were involved.
3.6. Sample Collection
- Using bronchoscopy BAL (bronchoalveolar lavage) technique, normal saline was used to lavage the bronchoalveolar region. The specimen was then placed in a sterile container. Only one specimen was collected from each patient. After collection, VTM was added as soon as possible. The volume of VTM that was added and the sample collected was equal. Patient’s number, age, gender and whether from ICU or MV was written on the specimen container. The sample was then stored at 4°C for transportation to KEMRI Nairobi for analysis.
3.7. Laboratory Analysis
3.7.1. RNA Extraction (QiaAmp RNA Mini Kit)
- The primary samples were frozen at -80°C to -40°C. 350 µl of lysis buffer was added to the sample aliquots. 350 µl 70% ethanol was then added to the sample and mixed well by pipetting three times. 700 μl of sample was placed on the spin column and centrifuged for 30 seconds at 13000 rpm, the supernatant was discarded. 700 µl of RW1 buffer was added to spin column and centrifuged for 30 seconds at 13000 rpm and the flow-through liquid discarded. This is followed by adding 500 µl of buffer (RPE) to spin column and centrifuged for 2 minutes at 13000 rpm. After discarding the supernatant, the spin column is placed in clean 2 ml tube and centrifuged for 1 minute at 13000 rpm. This is then placed in another clean 1.5 ml tube. 30-50 µl of RNase free water was finally added onto the membrane of the column and centrifuged for 1 minute at 13000 rpm. RNA will now be at the bottom of the tube.
3.7.2. DNA Extraction (QiaAmp DNA Mini Kit)
- 1. 20μl of proteinase K was placed on the bottom of a 1.5 ml microcenrtifuge tube.2. 200μl of the specimen was added to the microcenrtifuge tube. 3. 200μl AL buffer was then added and vortexing for 15seconds to ensure sufficient mixing and efficient lysis.4. This was followed by 10 minutes incubation at 56°C, this ensures maximum DNA yield after lysis for 10 min at 56°C. 5. 200μl of 96–100% ethanol was then added to the sample, and mixed by vortexing for 15 seconds. 1.5 ml microcenrtifuge tube was then centrifuged to get rid of drops in the lid.6. The cap was closed and centrifuged at 6000 x g (8000 rpm) for 1 minute and placed in a clean 2 ml collection tube.7. The Mini spin column was opened and 500 μl of AW1 buffer was added without wetting the rim. The cap was closed and centrifuged at 6000 x g (8000 rpm) for 1 min.8. The QIAamp Mini spin column was carefully opened and 500 μl of AW2 buffer added without wetting the rim. The cap was closed and centrifuged for three minutes at 20,000 x g; 14,000 rpm.9. The column was then placed in a new 2 ml collection tube and centrifuged for one minute at maximum speed.10. The QIAamp spin column was finally placed in a clean 1.5 ml microcenrtifuge tube. The collection tube containing the filtrate was discarded. The Mini spin column was carefully opened and added 200 μl AE buffer or distilled water. This was Incubated at 15–25°C for 1 minute and then centrifuged at 6000 x g (8000 rpm) for 1 minute. Elution was done with 200 μl off Buffer AE.It is recommended that elution be done in Buffer AE and stored at –30 to –15°C.
3.7.3. Preparation of Master Mix
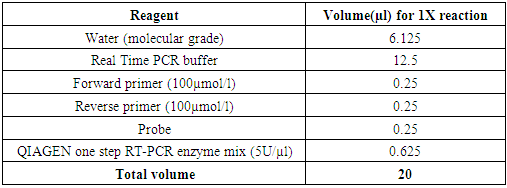
- Preparation of the master mix was done by adding the real time PCR components as shown on table 1 below in an Eppendorf tube before dispensing on Applied Bio systems PCR 96-well plate for the real time PCR to be carried out. The real-time PCR was carried out on AB 7500 Real Time Fast thermocycler, microcenrtifuge tubes and mixing gently by pipetting the master mix up and down ten times.
|
|
3.8. Data Analysis
- Data analysis is the process of data editing, coding and tabulation to reveal descriptive and inferential statistics. Descriptive statistics such as percentages, mean and standard deviation was used to analyze quantitative data. SPSS was used in entry, coding and also to analyze the data. The data was log-transformed to be able to have normal distribution and tabulated as mean±standard deviation. The results were compared based on whether in ICU or on ventilator support. This was also done based on gender of the study subjects using ANOVA with Bonferroni’s post-test using GenStat Release 14.1 (PC/Windows). Data presentation was done using graphs, pie charts, tables and figures.
3.9. Ethical Approval
- All patients that were recruited in this study gave informed consent that was written. Approval for this study was obtained from the Institutional research and ethics committee (IREC) of Moi University (School of medicine) and Moi teaching and referral Hospital. The data obtained from this study was confidential. Reference: IREC 2016/108, Approval Number: 0001807.
4. Results
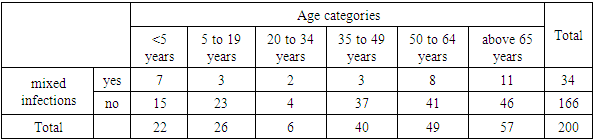
- The data was analysed to address the objective of the study. 200 samples were collected, out of these 82 samples (41%) were from ICU and 118 (59%) were from MV.
4.1. Real Time PCR Images
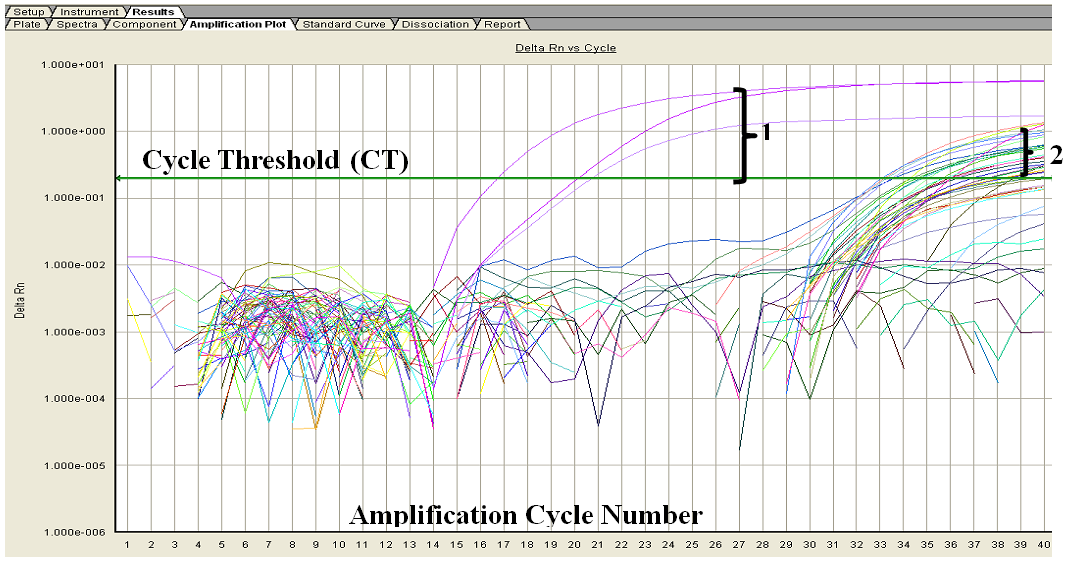 | Figure 1. Showing representative Real time PCR results. 1 – Represents the positive control results curves, while 2 indicates the representative field study samples results curves |
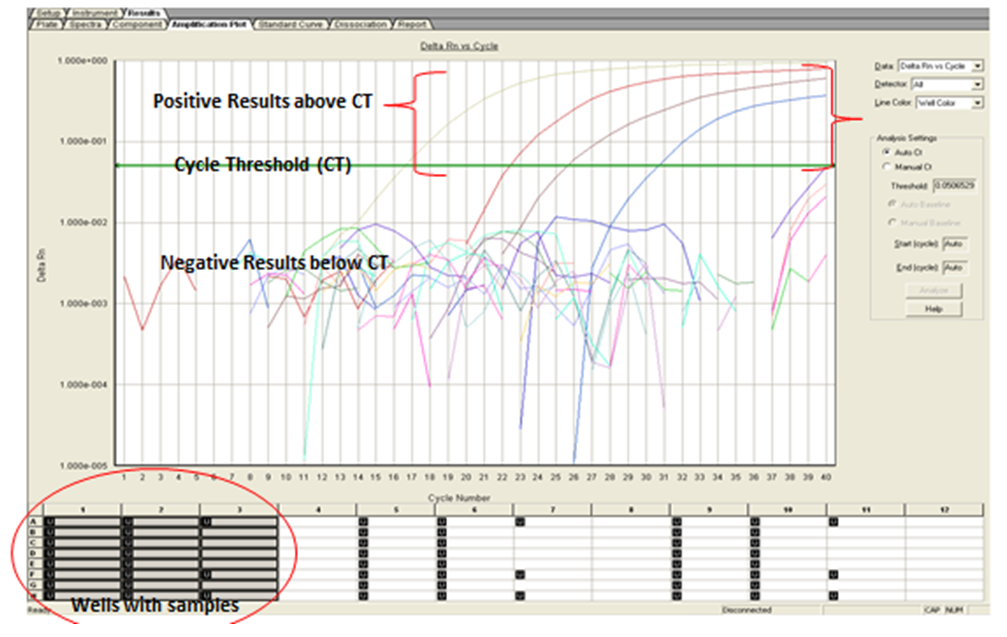 | Figure 2. Real time PCR image showing positive results above CT and negative results below CT |
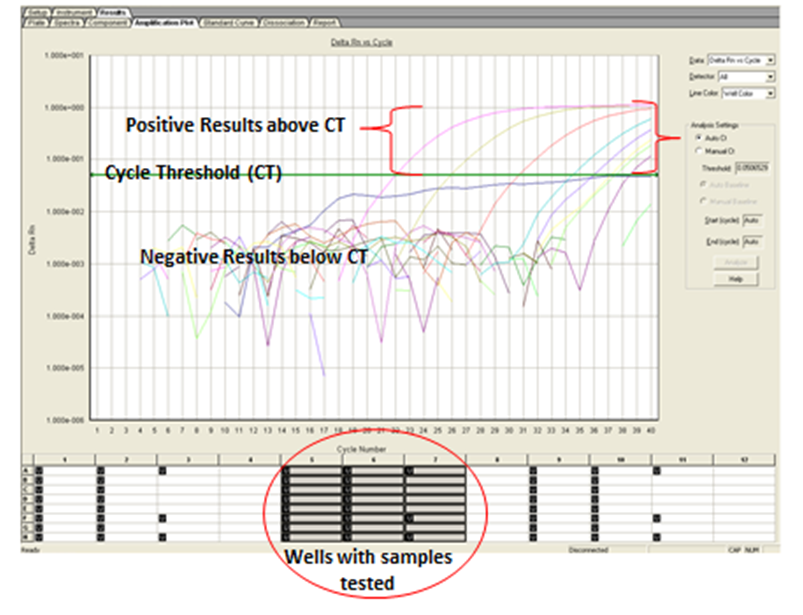 | Figure 3. Real time PCR image showing positive results above CT and negative results below CT |
4.2. Demographic Features of the Population Studied
- Gender and age of the population studied was tabulated to aid in investigating differences in prevalence of these infections in the various age groups and gender.
4.2.1. Age
- In this study, majority of the population studied were >65 years. This was 28.8% of the population studied. However those between 50- 64, 35-49, 5-19 and <5 years were 24.2%, 20%, 13% and 11% respectively. 3% of the population studied was between 20 to 34 years. The relevance of this age categories is because many studies have been done on children and the elderly hence it is important understand the distribution of these infections across all age groups.
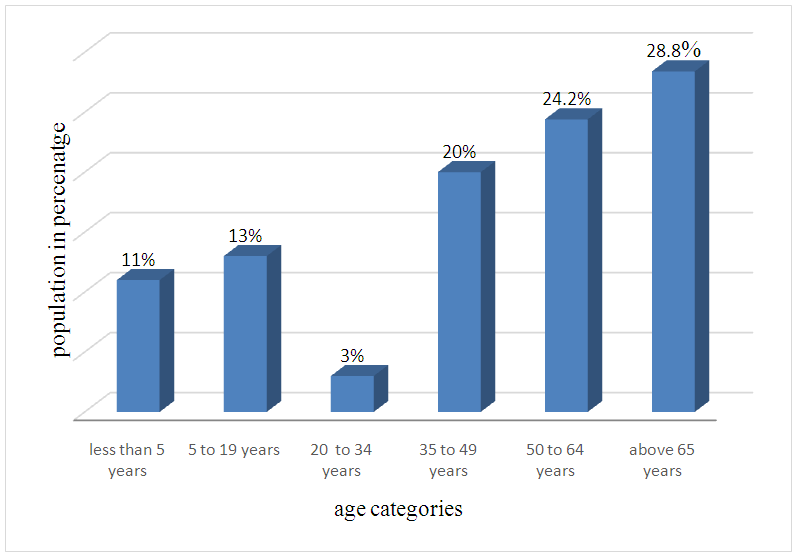 | Figure 4. Population of age categories in the study |
4.2.2. Gender
- Out of the 200 samples, 115 (57.5%) were male while the female were 85 samples (42.5%).
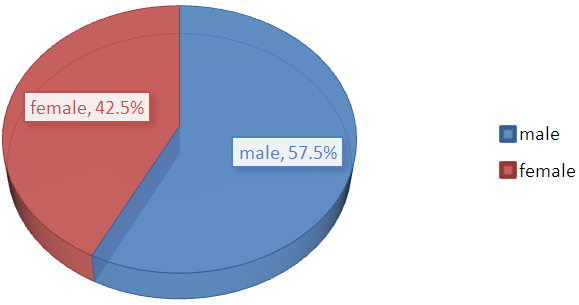 | Figure 5. Gender of the study population |
4.2.3. Specific Prevalence of Coinfections in the ICU and Mechanical Ventilation
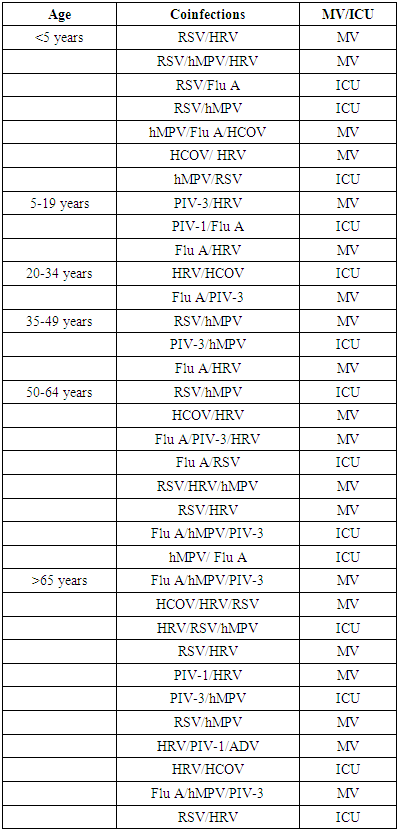
- The study also targeted at the prevalence of coinfections in the population studied. From the findings, it is clear that there were a significant prevalence of at least one virus in the samples analysed.There was high prevalence of coinfections as shown in figure 5 in ICU and MV by Influenza A + PIV-3 + hMPV at 4% and 3% respectively. This was followed by RSV + hMPV + HRV in MV at 3%. Both RSV + Influenza A and RSV + hMPV recorded a prevalence of 1% in ICU. HRV + Adenovirus and HRV + PIV-1 had a prevalence of 1% in MV and ICU respectively while HCOV+HRV +RSV and PIV-2 + hMPV + Influenza A recorded a prevalence of 2% in MV.
|
|
5. Discussion
- In the current study there was high prevalence of coinfections in ICU and MV by Flu A + Para 3 + HMPV at 4% and 3% respectively. This was followed by RSV + hMPV + Human Rhinovirus in MV at 3%. Both RSV+ Influenza A Virus and RSV+ hMPV recorded a prevalence of 1% in ICU patients. Co-infections were more common in age groups 50-64 years and those above 65 years. There were also significant levels of co-infections in patients below 5 years. RSV was found to be the most co-infected with other viruses in age group below five years. Rhinovirus was also found to be the most co-infected with other viruses in study subjects above 65 years and 50-64. In a study performed in Netherlands where five ICUs were involved from 2013 to 2014, 1,499 patients were studied. There was infection with at least one virus in 18% of the samples analyzed. Two viruses were detected in 17 patients. Three viruses were present in two samples. PIV-3 was the common with a prevalence of 5.7%, 1.1% (17) had an infection with Influenza Virus.Factors influencing the occurrence of these viruses are similar. Most of the respiratory viruses are transmitted directly through human contact, aerosol transmission or indirectly through fomites The common demographic factors associated with these viruses have been reported in various studies and include age, sex and preexisting health conditions. Additionally, host attributes and behaviors, comprising household overcrowding, daycare attendance, birth during the seasonal peak of infection, lower parental education level, inadequate hygiene, and lower breastfeeding rates, have been suggested to affect the distribution of these viruses [43].
6. Conclusions
- There was a high prevalence of coinfections in both ICU and MV. Co-infection combination of Influenza A Virus + PIV-3 + hMPV had the highest prevalence followed by RSV + hMPV + HRV. Co-infection combination of HRV+ Adenovirus and Rhinovirus + Parainfluenza had the lowest prevalence. Co-infection combination of RSV+ Influenza A Virus; RSV + hMPV; corona +HRV+RSV and PIV-2 + hMPV + Influenza A Virus was also noted.
7. Recommendations
- Coinfections should be closely monitored especially in mechanical ventilation in order to understand the impact of ventilator support on infection rates by these viruses.
Conflict of Interest
- The author(s) declare that there are no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML