-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2021; 11(2): 33-39
doi:10.5923/j.microbiology.20211102.01
Received: Oct. 7, 2021; Accepted: Oct. 25, 2021; Published: Dec. 29, 2021

Effects of Cultural Conditions on the Production of Extracellular Protease by Bacillus circulans Isolated from Dried Fish
Tahsina Jainab1, Sharmin Sultana2
1Faculty of Basic Medical and Pharmaceutical Sciences, University of Science and Technology Chittagong, Chittagong, Bangladesh
2Department of Microbiology, University of Chittagong, Chittagong, Bangladesh
Correspondence to: Tahsina Jainab, Faculty of Basic Medical and Pharmaceutical Sciences, University of Science and Technology Chittagong, Chittagong, Bangladesh.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Proteases are one of the important groups of enzyme and are attracting global enzyme market as well as biotechnological application. In this study, the effects of cultural condition for the production protease from Bacillus circulans, isolated from dried fish were carried out. Protease activity was screened on the basis of their ability to hydrolyze skimmed milked casein, egg albumin and gelatin. Highest protease achieved from the isolate after 48 hours of incubation at 37°C with pH 8, indicating the alkaline source of enzyme. Protease production specifically depends on the medium containing glucose as a carbon source and gelatin as nitrogen source at shaking condition. During enzyme substrate reaction, maximum protease activity was achieved at 40°C and pH 7.0 in the presence of 1.5% casein used as substrate. The protease has a specific activity of 966.03 U/ml at 60% saturation of ammonium sulfate. Finally, tyrosine and cysteine were found when end product was separated by using thin layer chromatography (TLC). The result of the present study might be helpful for large-scale production of extracellular proteases.
Keywords: Isolation, Bacillus circulans, Dried fish, Extracellular protease, Culture conditions, Amino acid
Cite this paper: Tahsina Jainab, Sharmin Sultana, Effects of Cultural Conditions on the Production of Extracellular Protease by Bacillus circulans Isolated from Dried Fish, Journal of Microbiology Research, Vol. 11 No. 2, 2021, pp. 33-39. doi: 10.5923/j.microbiology.20211102.01.
Article Outline
1. Introduction
- Proteases are single class of enzymes, constitute one of the most important groups of enzymes both industrially and academically. Protease hydrolyses peptide bonds in aqueous solutions and synthesizes them under non-aqueous conditions [1]. They are produced by various plants, animals and microorganisms sources, the latter are most widespread in nature, and are preferred owing to their fast growth, the limited space required for their cultivation. Though a wide variety of microorganisms are capable of producing these enzymes both intracellularly and extracellularly, the bacterial proteases are the most significant compared with animal and fungal proteases [2]. Alkaline proteases, which work optimally in alkaline pH are the main enzymes among proteases and constitute 60 to 65% of the global industrial enzyme market [3-4]. The major drawbacks of enzymes recovered from thermopiles are that enzymes from alkalophiles confer stability in a wide pH range but are also usually thermolabile that affect the stability at alkaline pH [5]. Of all proteases, alkaline proteases produced by Bacillus species are of significant importance in detergent industry due to their high thermal and pH stability [6]. Proteases are one of the most important groups of industrial enzymes used in the detergent, food, pharmaceutical, chemical, lather, paper and pulp and silk industries. Moreover, they are used in the food industry in meat tenderization processes, peptide synthesis, infant formula preparations, baking and brewing [7]. Besides, broad antimicrobial activity against E. coli indicated the use of the protease as an antibiotic for many infection and diseases [8].For the above concern the present study was conducted to study the effects of cultural conditions on the production of extracellular proteases from bacteria and partial purification of it.
2. Materials and Methods
2.1. Microorganisms
- Dried fish sample was used for the isolation of protease producing microorganisms. Different rotten dried fish were collected aseptically in sterile plastic zipper bag separately. In this study, enrichment media technique and gradual dilution technique were used for the isolation of the organisms.
2.2. Screening of the Isolates
- After isolation, primary screening and secondary screening were done for protease production. Primary screening was done by using enrichment technique followed by three method- Boiled egg albumin degradation, Skimmed milk casein hydrolysis and Gelatin hydrolysis. Then the organisms were examined for secondary screening, where the isolates were examined for the protease activity in liquid medium such as (i) Peptone 2%, dextrose 2%, yeast extract 1% [9], (ii) Tryptone 1%, dextrose 0.1%, yeast extract 0.5% [10], (iii) Gelatin 1%, glucose 1%, yeast extract 0.2%, K2HPO4 0.3%, KH2PO4 0.1%, MgSO4 trace [11] were used.
2.3. Measurement of Enzyme Activity
- Protease activity was determined by the modified method of Hayashi et al., 1967 [12] as followed by Meyers and Ahearn, 1977 [13]. In this method 3ml crude enzyme solution was incubated with 3ml of citrate phosphate buffer and 3ml of 1% casein at 35°C in a water bath. After that the reaction was stopped by adding 5 ml of 20% TCA. Then it was allowed to stay for one hour and filtered by Whatman (grade 40) filter paper (Ashless). This filtrate was used as enzyme substrate mixture for the determination of enzyme activity. For this reason 1 ml of enzyme substrate mixture, 2ml of 20% NaCO3 and 1 ml of Foin Ciocalteu Reagent were taken into a test tube and mixed well and waited for 30 minutes. Then 6 ml of distilled water was added to it and absorbance of the solution was measured at 650 nm in spectrophotometer. Enzyme activity was calculated from a standard curve plotted from known concentration of tyrosine. One unit (U) of enzyme activity represents the amount of enzyme required to liberate 1µg of tyrosine/ml under standard assay conditions.
2.4. Biomass Yield
- Bacterial biomass was determined by measuring the absorbance at 600nm [14].
2.5. Optimization of Culture Conditions
2.5.1. Effect of Incubation Time, Temperature and Medium pH
- In this study the effect of culture conditions was carried out at different incubation time (24, 48, 72 and 96 hrs.), temperature (10°C, 27°C, 37°C and 45°C) and pH (5.0, 6.0, 7.0, 8.0 and 9.0). The effect of different cultural conditions on biomass characteristics, biomass yield and protease production were recorded.
2.5.2. Effect on Carbon and Nitrogen Sources
- For this purpose four carbon (glucose, fructose, lactose starch), four organic nitrogen (peptone, gelatin, tryptone, bean) and two inorganic nitrogen [NaNO3, (NH4)2HPO4] sources of different concentrations (0.5%, 1.0%, 1.5%, 2.0% and 2.5%) were added to the medium separately and the effect of this carbon and nitrogen sources on the production of protease, biomass characteristics and biomass yield were recorded.
2.5.3. Effect of Stationary and Shaking Condition on the Production of Proteases
- To determine the effects of stationary and shaking conditions for the production of extracellular proteases, broth medium were incubated in both conditions keeping all other experimental conditions at optimum.
2.6. Optimization of Crude Enzyme Activity
- To optimize the crude enzyme activity, the factors involved during enzyme substrate reaction were taken into concern for further study. For this reason several factor such as temperature (35 to 55°C), pH (5.0 to 8.0), incubation time (50, 60, 80 and 90 minutes), substrate concentration (0.5, 1.0, 2.0, 2.5 and 3.0% of casein solution) and substrate specificity (casein, egg albumin, BSA and gelatin) observed.
2.7. Enzyme Purification
- The isolate was cultured in a 250 ml. Erlenmeyer’s flask using 100 ml. of optimized broth medium, and incubated at their optimum environmental conditions. After centrifuging the culture broth at 8000 rpm for 15 minutes at 4°C, powdered ammonium sulfate was slowly added to the cell free supernatant fluid at 50% saturation with constant stirring at 4°C and the mixture was left overnight at 4°C. The precipitates formed were collected by centrifugation at 12,000 rpm for 20 minutes at 4°C and dissolved in 6 ml of 0.1M sodium phosphate buffer pH 7.0. Separate fractions were obtained by precipitation with 60, 70 and 80% saturation of ammonium sulfate. The enzyme activity was determined for each separate fraction.
2.8. Determination of Amino Acid by Thin Layer Chromatography
- Amino acids are released by the hydrolysis of the protein with protease of our isolate were identified by Thin Layer Chromatography (TLC). Chromatograms were developed by the ascending method with TLC plate as the stationary phase and n-butanol: water: acetic acid (4:1:1) as solvent. Amino acids are detected by spraying with 0.25% ninhydrin in acetone [15].
3. Results and Discussion
- By using enrichment techniques four bacterial isolates were isolated from spoiled dried fish samples.
3.1. Screening and Identification of Selected Isolates
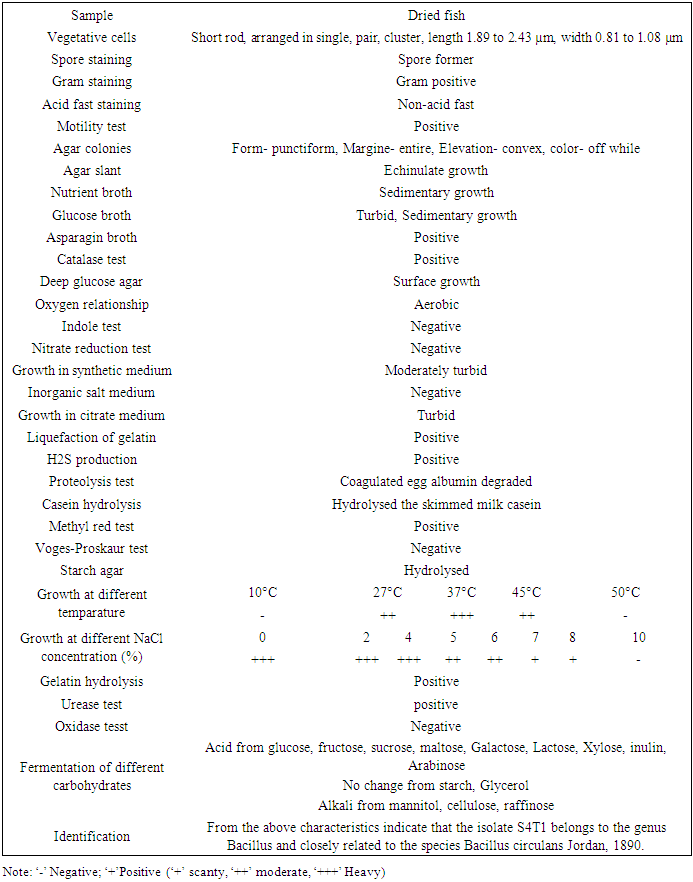
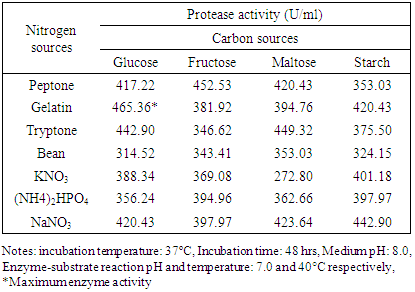
- All of these isolates were primarily screened by protein hydrolysis method and found that two bacterial strains were vigorously hydrolyzed the egg albumin, skimmed milk casein and gelatin. The isolates showed clear zone of hydrolysis in casein agar plate and gelatin agar plate after 24 hours of incubation at 37°C. Complete degradation of egg albumin was observed after 7 days of incubation at 37°C. Both the isolates were allowed to grow in three liquid media and S4T1 showed the maximum enzyme activity in Gelatin-yeast extract-glucose containing broth medium (Table 1). The bacterial isolate, S4T1 was the potent protease producers in the liquid medium that was finally selected for further study.
|
|
3.2. Optimization of Different Cultural Conditions
- The production of enzymes from microorganisms is strongly influenced by the composition of the medium, temperature, pH and the length of incubation. In order to go well with for high secretion of protease, proper combination of various cultural conditions can be established for B. circulans.
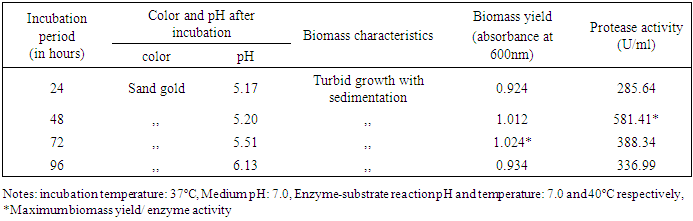
3.2.1. Effect of Incubation Time
- In this test to optimize the incubation time, protease production increased gradually 24 to 48 hours at which it was maximal, at 581.41 U/ml then decreased with time (Table 3). Similar study was also observed for bacteria with other research article [17].
|
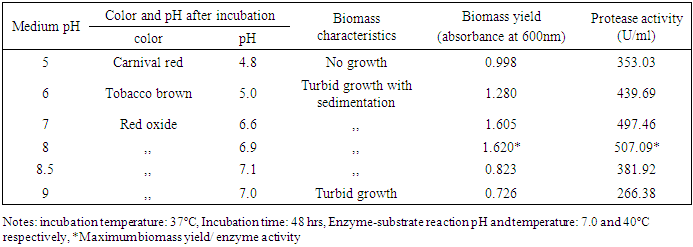
3.2.2. Effect of Medium pH
- Microorganisms are sensitive to the changes in hydrogen ion concentration of their environment. To determine the optimal pH for the protease production, Bacillus circulans (S4T1) was cultivated over the pH range 5-9 at room temperature. The highest protease activity was found at pH 8 (507.09 U/ml) (Table 4). Production of protease at pH 8.0 by Bacillus licheniformis was reported previously [18]. But most of the Bacillus sp. reported has optimum pH from 7.0 to 11.0 for the production of protease [19-20].
|
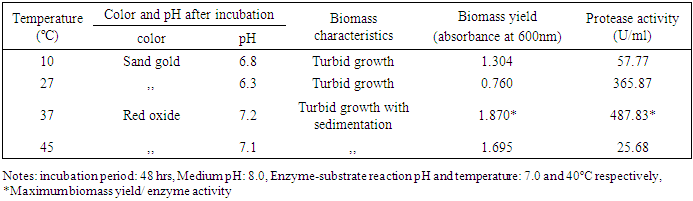
3.2.3. Effect of Temperature
- The highest protease production (487.83 U/ml) and maximum biomass yield was recorded at incubation temperature 37°C with turbid growth by the isolate S4T1 (Bacillus circulans) (Table 5). Usually the bacterial isolate prefer 37°C for maximum production of protease which are in concurrence with several workers [17,19-22].
|
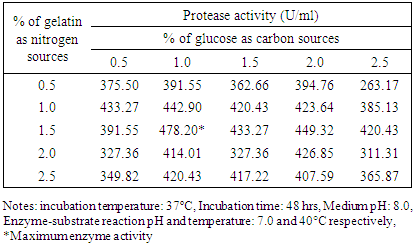
3.2.4. Effects of Carbon and Nitrogen Sources
- The isolate S4T1 (Bacillus circulans) showed maximum enzyme activity in 1% glucose and 1.5% gelatin containing media (Table 6 and Table 7).
|
|
3.2.5. Effect of Stationary and Shaking Condition
- Stationary and shaking conditions have marked influence on proteases production. The isolate S4T1 (Bacillus sphaericus) showed maximum protease production (616.21 U/ml) at shaking condition (110 rpm). Maximum protease production at shaking condition was reported by sever authors [25-27]. Similar observation was also recorded by the isolate S4T1.
3.3. Factors Involved on Enzyme Activity
- Enzyme activity depends upon the pH, temperature, incubation time, substrate concentration, substrate specificity and many other factors. So, it is necessary to find out the limiting factor for maximum activity of proteases. The protease activity of crude enzyme of bacterial isolate S4T1 (Bacillus circulans) increased gradualy to a maximum level of 529.55 U/ml at 70 minutes of incubation during enzyme substrate reaction; thereafter, activity decreased gradually with the rise of incubation time (Figure 1a). The isolate was allowed to react with different substrate concentrations (0.5 to 3.0%) and maximum activity of 519.92 U/ml was found with 1.5% casein was used as substrate (Figure 1b).Temperature and pH are also most important limiting factors, which markedly influenced enzyme activity. Maximum protease activity of crude enzyme extract of bacterial isolate S4T1 (Bacillus circulans) was recorded 548.81 U/ml at reaction mixture temperature 40°C and pH 7.0 (Figure 1c).Similar work at acid to neutral pH and different temperature was also done by several workers [20,28].
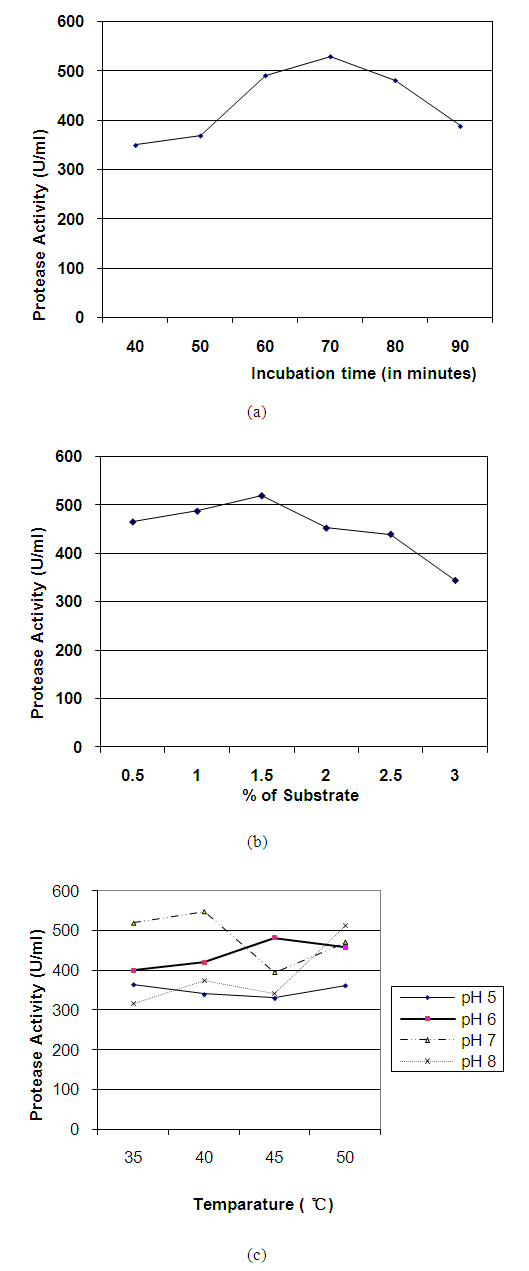 | Figure 1. (a) Effects of incubation time (b) substrate concentration (c) pH and temperature on protease (crude) activity of Bacillus circulans (S4T1) |
3.4. Enzyme Purification
- Enzyme was partially purified by solubility method using 50 to 80% ammonium sulfate. The yield of maximum protease activity of partially purified enzyme of the isolate S4T1 (Bacillus circulans) was recorded at 60% ammonium sulfate saturation with a total activity of 966.03 U/ml (Figure 2).
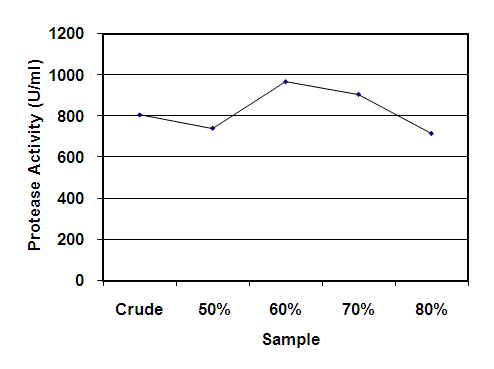 | Figure 2. Effects of crude and partially purified enzyme of Bacillus circulans (S4T1) |
3.5. Determination of Amino Acid
- The ends products of enzyme activity were determined by thin layer chromatography (TLC). Under optimum condition, the released amino acids after hydrolysis of proteins were detected. Five spots with Rf value of 0.09, 0.36, 0.62, 0.84 and 0.94 were recorded with the isolate S4T1 (Bacillus circulans). Two spots (Rf value of 0.36 and 0.94) were confirmed with the authentic amino acids tyrosine and cysteine (Figure 3).
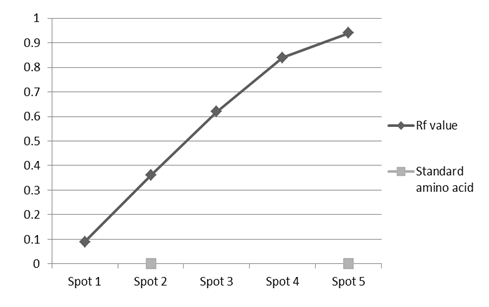 | Figure 3. Rf value of different amino acids of Bacillus circulans (S4T1) |
4. Conclusions
- B. circulans that is isolated from dried fish is the potential microbes for extracellular protease production, and various environmental and nutritional factors have significant effects on their growth and protease production. In this study, we found the most active enzyme fractions were obtained at 40°C with pH 7.0 and 60% ammonium sulfate saturation, with specific activities from 616.21 to 966.03 U/ml when 1.5% casein used as substrate. The extracellular protease produced by B. circulans exhibits optimal activity at pH 8.0 indicating the alkaline source of the enzyme. The protease production was dependent on the carbon and nitrogen source in the culture media of the organism. The potential application of the enzyme with recyclable capacity was indicated by their ability to hydrolyse the main by-product of the brewery industry. By implementing the optimized media composition and cultural conditions, the bacterial strain would produce significant amount of protease which could find applications in large scale in industry and biotechnology. Further work with the split of many other factor and interactions of each factors may provide clear picture about maximum production of protease by the selected isolates. More investigations on the bacteria can likely reveal their potentiality as a source of protease which could provide important future benefits to industry.
ACKNOWLEDGEMENTS
- The research work was done at the department of Microbiology in the University of Chittagong, Bangladesh. The Authors are grateful to the management and staff of this department and also grateful to author/ editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML