-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2020; 10(3): 71-79
doi:10.5923/j.microbiology.20201003.02
Received: Nov. 18, 2020; Accepted: Dec. 8, 2020; Published: Dec. 15, 2020

Biopreservation against Fungi Contamination of Traditional Food (Iassa) Produce by Fermentation of Hibiscus sabdariffa Seeds
Tchikoua Roger, Edzili Awono A. T., Youté Fanche S. A., Essia Ngang J. J.
Department of Microbiology, University of Yaounde 1, Yaounde, Cameroon
Correspondence to: Tchikoua Roger, Department of Microbiology, University of Yaounde 1, Yaounde, Cameroon.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Uncontrolled fermentation is a method of transformation which can expose food to risks of contamination by moulds and mycotoxins. Food contaminations represent a potential threat to the health of consumers. In the far North of Cameroun, Iassa obtained by spontaneous fermentation of Hibiscus sabdariffa grains belongs to this food category. This study was aimed at preserving the safety quality of Iassa against fungal growth during fermentation by using lactic acid bacteria (LAB). To carry out this work, moulds and LAB were first isolated from Iassa samples collected in the locality of Gamboura (Cameroon). This was followed by the production of Iassa at the laboratory which was inoculated with moulds and LAB, and the entire batch was left to ferment for 120 hours at 25°C. The antifungal activity of LAB and titratable acidity were evaluated during fermentation. The results showed that, Iassa is a favourable food to the development of moulds. High contamination of this product by moulds of the genus Aspergillus and Fusarium were observed. Generally, this contamination ranged from 2.9Log10CFU/g to 6.9Log10CFU/g. Among the isolated LAB, Lactobacillus brevis and Lactobacillus paracasei were identified as the best LAB with good antifungal activity. Lactobacillusbrevisinhibited completely the growth of Aspergillus in Iassa after 96 hours of fermentation. During this assay, a high growth of Lactobacillus brevis varying between 12.0 and 13.3 Log10CFU/g was also observed. Unlike Lactobacillus brevis, Lactobacillus paracasei produced a high quantity of acidity evaluated at 0.9%. From this work, these bacteria can be used as a LAB starter to ensure safety of the Iassa against moulds after 96 hours of fermentation.
Keywords: Fermentation, Hibiscus sabdariffa, Antifungal activity
Cite this paper: Tchikoua Roger, Edzili Awono A. T., Youté Fanche S. A., Essia Ngang J. J., Biopreservation against Fungi Contamination of Traditional Food (Iassa) Produce by Fermentation of Hibiscus sabdariffa Seeds, Journal of Microbiology Research, Vol. 10 No. 3, 2020, pp. 71-79. doi: 10.5923/j.microbiology.20201003.02.
Article Outline
1. Introduction
- In many African countries, the seeds of Hibuscus sabdariffa are used as condiments during the preparation of traditional food. The name of these spices varies from one country to another. In Niger, Mali, Sudan and Cameroon this condiment is respectively called Dawadawa botso, Datou, Furundu and Mbuja [1]. In the Far North of Cameroon (Gamboura), these same seeds of Hibuscus sabdariffa are used for the production of a fermented food called Iassa. This product, little known beyond this particular area where it is produced [2], is a very popular food and widely consumed throughout the year by local populations. It is consumed as a sauce accompanied with red millet or corn fufu on special occasions. According to local customs, Iassa must be automatically consumed by women who have just given birth in order to restore their lost strength during childbirth and for the maintenance of the baby during breastfeeding which can sometimes span over 2 years.Thus, due to the high protein content (39.5g/100g) of Hibiscus sabdariffa seeds, Iassa contributes significantly to the dietary needs of consumers. Unfortunately, studies carried out on fermented products obtained from Hibuscus sabdariffa seeds showed the bad sanitary quality of these products. Moreover, studies conducted on the hygienic quality of Soumbala, a condiment obtained by fermentation of the seeds of Hibiscus sabdariffa and Parkia biglobosa in Burkina Fasso have revealed the presence of the moulds in this food [4]. Likewise, the analysis of Mbuja consumed in the North region of Cameroon has shown the presence of spores of Bacillus cereus and enterococci [5]. In addition, traditionally fermented foods are generally contaminated with acid tolerant fungi species as Penicillium, Fusarium and Aspergillus [6]. Given the importance occupied by Iassa in the diet of these local populations and the potential dangers it represents, it would be wise to seek solutions to this problem of fungal contamination during spontaneous fermentation. However, the solutions to this problem should be part of the production system for this food. The use of lactic acid bacteria starters widely involved in traditional food fermentation has demonstrated their ability to inhibit the growth of moulds during fermentation [7,8] through the secretion of many antifungal compounds such as organic acids, diacetyl, bacteriocins, hydrogen peroxide, alcohols and reuterin [9]. In order to improve the safety of Iassa, the present study was aimed at evaluating the antifungal activity of lactic acid strains during fermentation of Iassa.
2. Material and Methods
2.1. Sample Collection
- The Iassa samples were collected in Gamboura, located in the Department of Mayo-Tsanaga in Cameroon and Mokolo (Far North) as its main town. A total of 30 samples of Iassa, weighing 6000g, were collected and stored in sterile plastic bags for various analysis. The figure 1 shows what the Iassa samples look like.
 | Figure 1. Sample of Iassa |
2.2. Iassa Production Investigation
- An investigation carried out with the producers made it possible to reproduce the Iassa production process in the laboratory. Firstly, Hibiscus sabdariffa seeds were washed and cooked on fire to make them tender. Then, these seeds were left to ferment in a container for 5 days at room temperature. This allows the bacteria to use by fermentation, the sugars present in the product. After this period, the fermented seeds are finely ground on stone and mixed with basic leachate obtained from the ash of dry cotton seeds. This step directs microbial metabolism towards intense proteolytic activity, promoting the production of metabolites such as various amino acids and ammonia. These changes contribute to the improvement of its organoleptic characteristics appreciated by consumers. The figure 2 gives the production process of the Iassa.
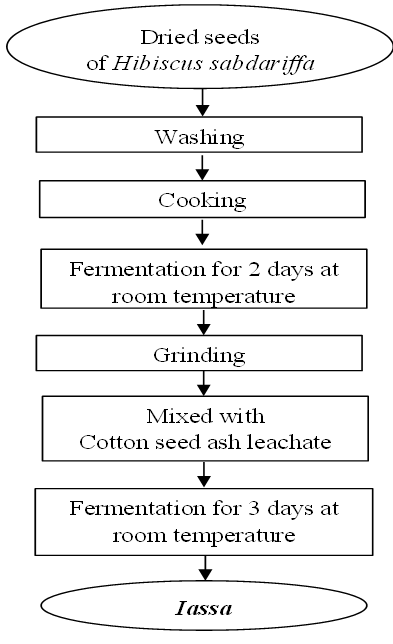 | Figure 2. The process of Iassa preparation |
2.3. Isolation of Microorganisms
- To isolated Lactic acid bacteria and mould, 10g of fermented Iassa sample was diluted in 90ml of sterile saline water (0.85% NaCl) to prepare the first dilution. 0.1 ml of each dilution was plated on the surface of Potato Dextrose Agar (PDA) to isolate mould and on De Mann, Rogosa and Sharpe Agar (MRS) supplemented with benzimidazole to isolate lactic acid bacteria. The MRS Petri dishes were incubated in a jar containing a candle for creation of anaerobic conditions, and all the dishes were incubated at 37°C for 24 at 48 hours. For the mould, PDA dishes were incubated at 25°C for 3 to 5 days [10,11].
2.4. Screening of Antifungal Lactic Acid Bacteria
- Firstly, the isolated moulds of Iassa, were cultivated on the PDA agar for 7 days until sporulation. The spores were then collected using a sterile platinum loop and introduced into 10ml of sterile physiological water. The stock solution obtained was mixed with tween 80 (0.1g/100ml). Dilutions of the stock solution were made and a count using the Malassez cell was performed to assess the concentration of the spores. Suspensions of 105CFU/ml were prepared and stored at 4°C. The lactic acid bacteria were streaked in the middle of Petri dishes containing MRS agar. The dishes were incubated for 48 hours at 37°C. After this time, the preparation was covered with 10 ml of Mueller Hinton medium (MH) contaminated with 105spores/ml of mould. Then, the entire preparation was incubated at 25°C for 48hours. The presence of an inhibition zone made it possible to select lactic acid bacteria which have antifungal activity [12].
2.5. Quantitative Test of Antifungal Lactic Acid Bacteria
- This test permitted to choose among the bacteria selected, those having the best diameter of inhibition. First, a well of 6mm was drilled in the centre of each Petri dish containing 20mL of MRS agar, using a sterile tip. Then, 20μl of a lactic acid bacteria inoculum of concentration 109CFU/ml was mixed with 30μl of the MRS agar and introduced using a micropipette into each well. After solidification, the dishes were incubated at 37°C for 48 hours. After incubation, each preparation was covered with 10mL of contaminated MH medium with a spore concentration estimated at 105spores/ml. The Petri dish was incubated at 25°C for 48 hours. The zone of inhibition formed was measured using a calliper and expressed in mm [13].
2.6. Characterization of Mould
- The isolated moulds were first cultured onto PDA-Chloramphenicol medium (250 mg/l) and incubated at 25°C for 7 days. The characterization was based on macroscopic studies on the appearance, colour and the texture of the mycelium. The Microscopic studies were also carried out on the shape of the vegetative thallus, conodiophore, and head morphology [10].
2.7. Molecular Identification of Active Lactic Acid Bacteria
- The pure strains of lactic acid bacteria isolated from Iassa were multiplied in an MRS broth at 37°C for 48 hours. The culture obtained was then centrifuged at 5000 rpm for 5 minutes. The pellet formed was collected for DNA extraction, which was carried out with the Qiaamp cador pathogen kit (qiagen kit supplied by Biozyme, Romania) according to the manufacturer’s instructions.The PCR amplification was perform with the primers LacF (5'-AGCAGTAGGGGAATCTTCCA-3') and LacR (5'-ATTCCACCGCTACACACATG-3') of 20 base pairs. These primers are specific to the group of lactic acid bacteria and have been amplified by the program proposed by Ponnusamy et al. [14] in a multigene thermocycler (mycycler thermal cycle, BIO RAD, Hercules, USA). The PCR reaction product was made up to a final volume of 50 μl by mixing 5 μl of MgCl2, 1 μl of dNTPs, 2.5 μl of each primer, 0.25 μl of Taq polymerase (Promega corp., USA) and 10 µL of DNA. The program used was as follows: initial denaturation at 94°C / 2 minutes; 30 denaturation cycles at 94°C / 15 seconds, hybridization at 51°C /15 seconds, elongation at 72°C /30 seconds; final elongation at 72°C/7 minutes [15]. To separate and characterize the PCR products, 10 µL of this product were migrated by electrophoresis with 2% agarose containing ethidium bromide (7g/L). The amplified DNA was sequenced by Base Clear Netherlands and the sequences were submitted to the NCBI for their identification and recorded in the online database of the NCBI.
2.8. Antifungal Activity of Lactic Acid Bacteria during the Fermentation of Iassa
2.8.1. Production of Iassa and Inoculation
- The seeds of Hibiscus sabdariffa which are used for the preparation of Iassa were first sorted, winnowed and disinfected in 2% aqueous solution of sodium hypochlorite (8°Chl) for one minute at room temperature in order to eliminate natural contaminants. The disinfected seeds of Hibiscus sabdariffa were then rinsed thoroughly with sterile distilled water (1/2, V/V) to remove traces of sodium hypochlorite. Subsequently, these seeds were cooked in a pressure cooker during 45 minutes. 200g of cooked Hibiscus sabdariffa seeds were inoculated with 2ml of inoculum of lactic acid bacteria and 2ml of mould spores with a concentration of 109 CFU/ml and 106 CFU/ml respectively. At the same time, control preparation was inoculated only with fungal spores. After this inoculation, 20g of the preparation were taken immediately for the enumeration of moulds, lactic acid bacteria and titratable acidity. The rest of the preparation was covered and incubated at 25°C for 2 days for the first fermentation. At the end of this fermentation, the seeds of Hibiscus sabdariffa were crushed aseptically and 20 g were again taken to carry out the same analysis mentioned above. The rest of the crushed seeds were added with cotton ash leachate (1/2, m/V). The wet paste obtained after the addition of leachate was fermented for 3 days at 25°C. Analyses were performed every 24 hours for the 3 days. The process for the preparation and inoculation of Iassa is shown in figure 3.
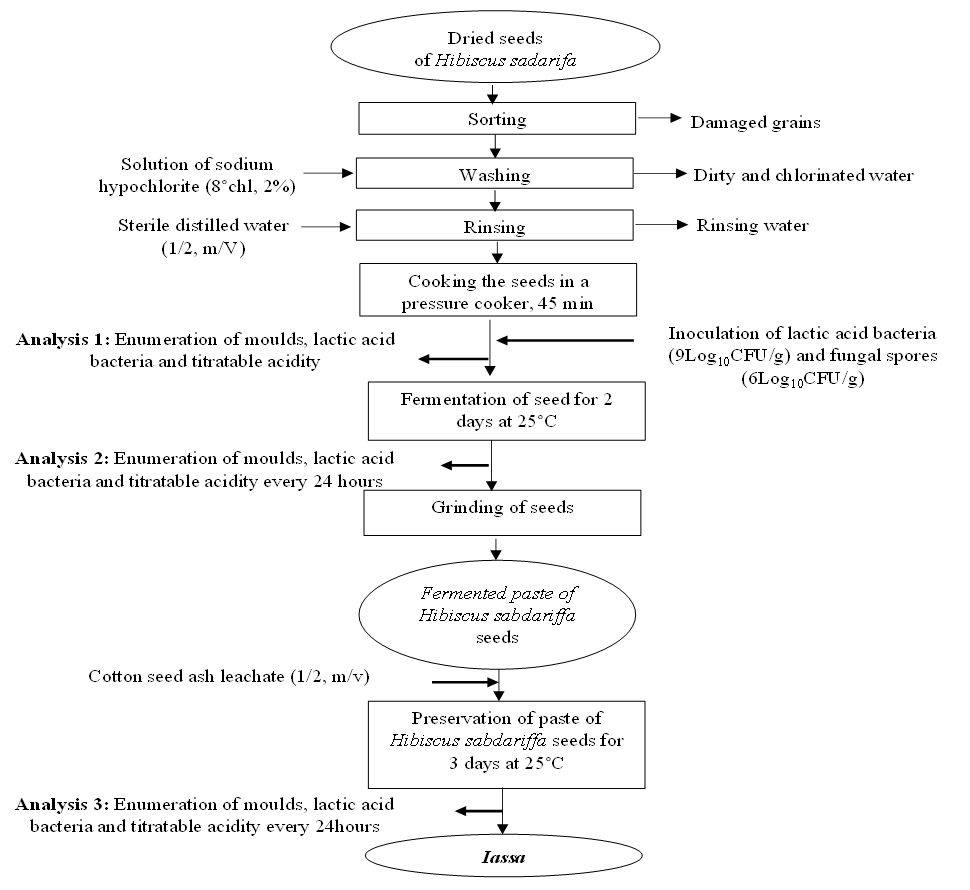 | Figure 3. Process for the preparation and inoculation of Iassa |
2.8.2. Enumeration of Microorganisms during the Fermentation of Iassa
- Lactic acid bacteria and moulds were first isolated from the dilutions as described earlier in this study. The microorganism count was done in Petri dishes containing between 30 and 300 colonies. The microbial loads were expressed in CFU/g using the formula below:
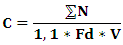 C: Microbial concentration (CFU/g);N: Total colonies counted on a Petri dish of two successive dilutions;Fd: Dilution factor of the smallest dilution;V: seed volume (ml).
C: Microbial concentration (CFU/g);N: Total colonies counted on a Petri dish of two successive dilutions;Fd: Dilution factor of the smallest dilution;V: seed volume (ml).3. Statistical Analysis
- The results obtained were analysed using Statgraphics 5.0 software for analysis of variances, calculation of means and standard deviations. Sigma Plot 11.0 software use for graphical representation of data.
4. Results and Discussion
4.1. Evaluation of the Fungal and Lactic Profile of Iassa
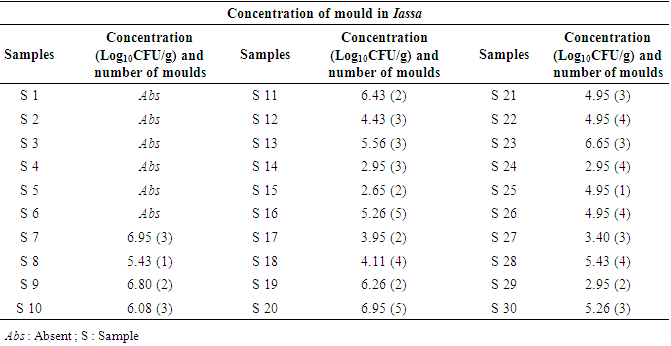
- After analysis, the results revealed the presence of LAB in the Iassa. The contamination of Iassa by moulds was also noted. 71 moulds were isolated from samples analysed, thus contaminating 80% of samples collected. The evaluation of the degree of contamination shows a strong contamination of certain samples of the Iassa with the concentrations of the fungal flora ranging between 2.9Log10CFU/g and 6.9Log10CFU/g. However, samples S7, S8, S9, S10, S11, S13, S16, S19, S20, S23 and S28 were the most contaminated with concentrations between 5.5 and 6.9 Log10CFU/g. The moderately contaminated samples made of S12, S17, S18, S21, S22, S25, S26, S27, S14, S24 and S29 with concentrations between 2.9 and 4Log10CFU/ml. While the samples ranging from S1 to S6 were not contaminated with mould. Table 1 shows the contamination rate of Iassa by moulds.
|
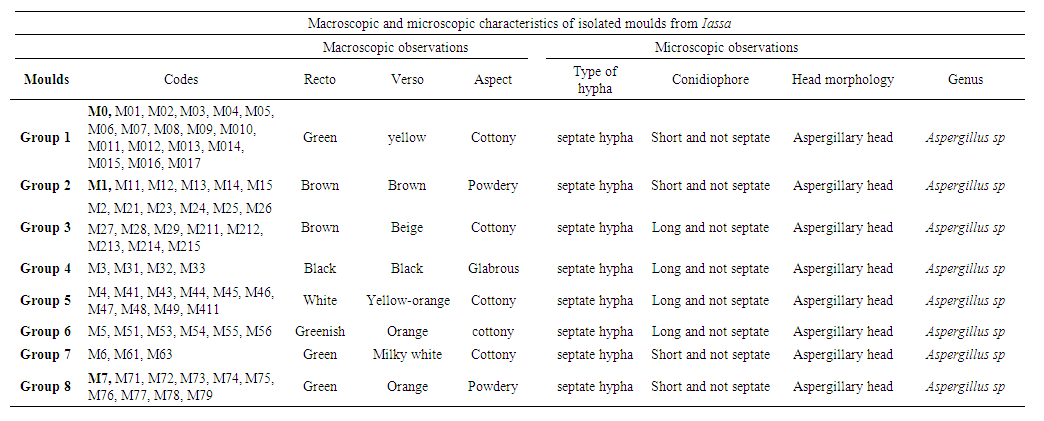 | Table 2. Macroscopic and microscopic characteristics of isolated moulds from Iassa |
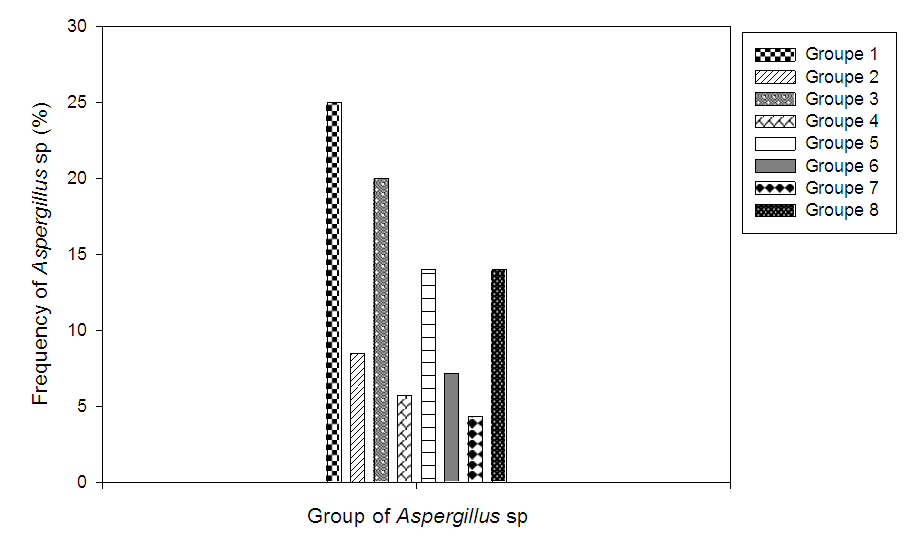 | Figure 4. Frequency of appearance of Aspergillus sp |
4.2. Screening of Active Lactic Acid Bacteria
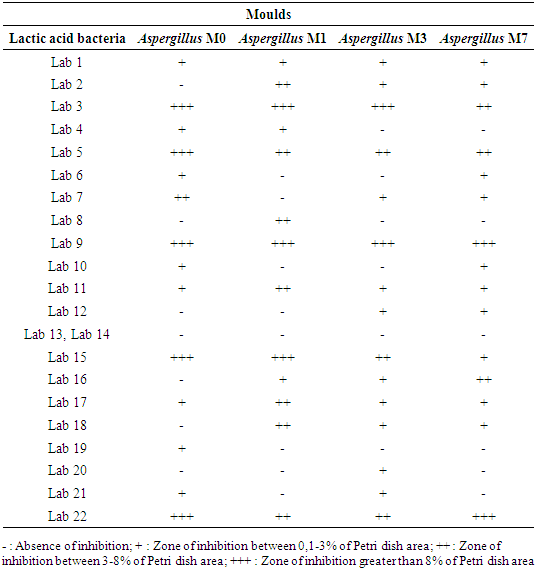
- After analysis, 53 LAB were isolated from the Iassa. The catalase tests, macroscopic and microscopic criteria, permitted to select and regroup these bacteria into 22 LAB. The presence of LAB in Iassa confirms the results of several authors who isolated LAB in foods obtained from the fermentation of Hibiscus sabdariffa seeds [4,5]. The confrontation of these LAB with Aspergillus revealed that out of the 22 lactic acid bacteria isolated, 20 LAB showed antifungal activity on at least one mould. The intensity of this antifungal activity varies from one LAB to another and from one mould to another. Among the 22 LAB tested, 05 showed an inhibition greater than 8% on the surface of the Petri dish. These were Lab3, Lab5, Lab9, Lab15 and Lab22. The LAB Lab2, Lab11, Lab17 and Lab18 had an inhibition zone between 3 and 8% on the surface of the Petri dish. In the rest of this study, the 05 LAB which exhibited the best antifungal activities were selected for the determination of their inhibition diameter. Table 3 shows the antifungal power of the 22 lactic acid bacteria retained after characterisation.
|
4.3. Determination of the Inhibition Diameter
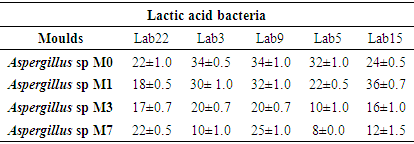
- In general, the LAB selected had antifungal activity on all of the moulds tested. However, this antifungal activity depended on the strain of LAB and the type of mould. It appears that Lab9 presented the best inhibitions on the moulds tested. It is followed by Lab3. However, Lab15 registered a greater inhibition diameter when it was tested on Aspergillus M1 (36 ± 0.7mm). The smallest inhibition was observed with Lab5 when it was tested on Aspergillus M7 (8 ± 0.0 mm). Given the efficiency of Lab3 and Lab9, they were chosen for the rest of the work. Table 4 shows the diameters of inhibitions induced by the different LAB selected.
|
4.4. Molecular Identification of Lactic Acid Bacteria
- At the end of agarose gel electrophoresis, the DNA of Lab3 and Lab9 had the same molecular weight (340 base pairs). After sequencing, Lab3 was identified as Lactobacillus paracasei with 97.73% and Lab9 lactic bacteria as Lactobacillus brevis with 97.54%. The studies of Swain et al. [19] have demonstrated the presence of Lactobacillus brevis in fruits, vegetables and plants. In addition, Lactobacillus brevis has been found in Mbuja, a condiment obtained from the seeds of Hibiscus sabdariffa [5] and in many non-alcoholic starch products based on cereals [20]. Lactobacillus paracasei has also been identified in Kefir, a food traditionally fermented in Brazil [21] and in cocoa beans fermented in Cameroon [22].
4.5. Antifungal Activity of Lactic Acid Bacteria during the Fermentation of Iassa
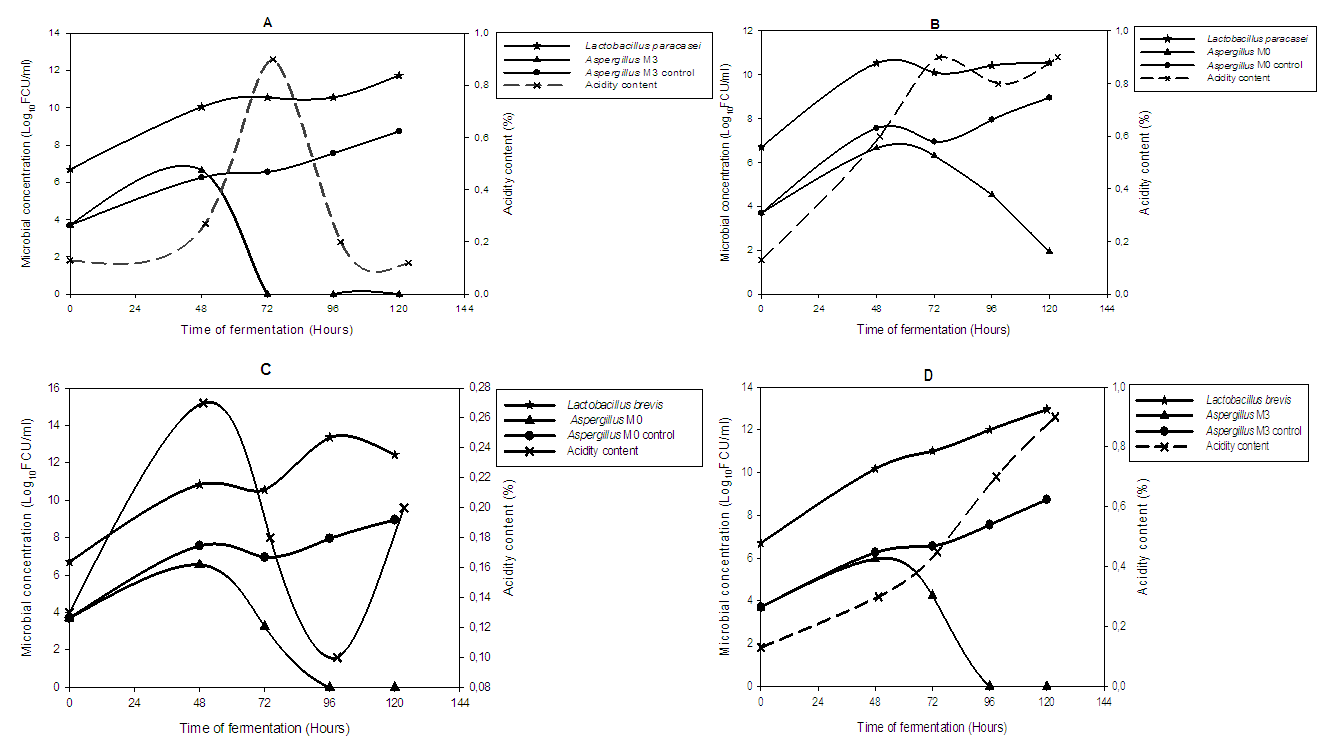
- The results of the antifungal activity of Lactobacillus paracasei and Lactobacillus brevis in the Iassa contaminated by Aspergillus M0 and Aspergillus M3 as well as the level of titratable acidity are shown in figure 5.
5. Conclusions
- The Iassa produced from the fermentation of the grains of Hibiscus sabdariffa presents strong contamination by moulds which are mainly composed of the genus Aspergillus. Nevertheless, one of the lactic acid bacteria isolated from Iassa, Lactobacillus brevis, completely eliminates contaminants such as Aspergillus M0 and Aspergillus M3 after 96 hours of fermentation. The use of this bacteria as a starter during fermentation makes it possible to control and eliminate the fungal contamination before the 120 hours of fermentation of Iassa. Thus, this controlled fermentation guarantees the safety quality of Iassa.
ACKNOWLEDGEMENTS
- Authors are grateful to the University of Yaounde 1 (Cameroon) for support in the form of infrastructural facilities made available for undertaking the present study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML