-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2020; 10(2): 55-58
doi:10.5923/j.microbiology.20201002.03
Received: Oct 3, 2020; Accepted: Nov. 2, 2020; Published: Nov. 15, 2020

Isolation and Identification of Pathogenic Bacteria from Fresh Fruits and Vegetables in Chittagong, Bangladesh
Baishakhi Biswas1, Md. Abul Kalam Azad1, Nurul Absar1, Saiful Islam2, Sabrina Amin1
1University of Science & Technology Chittagong, Department of Biochemistry and Biotechnology, Faculty of Basic Medical and Pharmaceutical Sciences, Foy’s Lake, Khulshi, Chittagong, Bangladesh
2Industrial Microbiology Research Division, BCSIR Laboratories Chittagong Chittagong, Bangladesh
Correspondence to: Md. Abul Kalam Azad, University of Science & Technology Chittagong, Department of Biochemistry and Biotechnology, Faculty of Basic Medical and Pharmaceutical Sciences, Foy’s Lake, Khulshi, Chittagong, Bangladesh.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Fresh fruits and vegetables are an essential part of people's diet all over the world. Since they are typically eaten raw and often without heat treatment or thorough washing, so they serve as vectors for the transmission of pathogenic micro-organisms associated with human diseases. This study aimed at isolating and identifying the pathogenic bacteria from selected popular fresh fruits and vegetables which are known as street food. A total of 7 fresh fruits and vegetables were collected from different open markets of Chittagong with aseptic condition. Selective and non-selective media were used to isolate and enumerate the bacteria. All the species were identified on the basis of morphology, biochemical tests (IMViC) and selective differential culture media. Among the samples Vibrio spp., Lactobacillus spp., Pseudomonas spp. and Salmonella spp. were dominant. All of fresh fruits and vegetables were heavily contaminated with coliform and fecal coliform (> 1100 CFU/100ml). Range of microbial count of guava was 2.1×104 CFU/ml, apple was 4.3×104 CFU/ml, tomato was 8.2×104 CFU/ml, cucumber was 5.0×104 CFU/ml, carrot was 1.3× ×104 CFU/ml, hog plum (Amra) was 7.5×104 CFU/ml. This results indicated that the serious concern for the people of Chittagong area as it can be a great risk for human health. So this results, suggested the necessity to create awareness about hygienic practices in selling and buying fresh fruits and vegetables.
Keywords: Pathogenic bacteria, Isolation, Identification, Fruits, Vegetable
Cite this paper: Baishakhi Biswas, Md. Abul Kalam Azad, Nurul Absar, Saiful Islam, Sabrina Amin, Isolation and Identification of Pathogenic Bacteria from Fresh Fruits and Vegetables in Chittagong, Bangladesh, Journal of Microbiology Research, Vol. 10 No. 2, 2020, pp. 55-58. doi: 10.5923/j.microbiology.20201002.03.
Article Outline
1. Introduction
- In recent years, outbreaks of human infections associated with the consumption of fresh or minimally processed fruits and vegetables have increased, despite their nutritional and health benefits [1,2]. Human disease outbreaks have been recognized as being caused by contaminated vegetable and fruits consumption, several studies have been published describing the bacterial contamination of intact vegetables and fruits in open markets [3]. Raw vegetables host a variety of pathogenic microorganisms that may be spread over the plants or occur in the plant tissues as micro colonies [2]. Some diverse factors may be affected the variation of microbial profile of vegetables including normal microflora of soil, animal manure derived flora, irrigation or sewage water, transportation and unconscious handling by retailers [Ref?]. Microbial contamination is commonly exposed to fruits and vegetables by contact with dirt, dust and water and by handling at harvest or during post-harvest processing [4,5]. Therefore, a wide range of microorganisms are present including plant and human pathogens. Due to lack of surveillance and inadequate screening of these raw vegetables in Chittagong region, most outbreaks have become undetected and there is very little information available in the literature so far [6].In another study, it was found that bacterial contamination of fruits and salad vegetables (tomato, cucumber etc.) was correlated with the fact that it is typically eaten without thermal treatment. A major contributing factor to contamination is use of untreated wastewater and manure as fertilizers for the production of fruits and vegetables [7]. In Bangladesh various fruits and vegetables are sold in open markets and most of them are eaten raw, which is suitable medium for bacterial contamination [8]. In developing countries like Bangladesh, both poverty and poor sanitation is common, faecal contamination of domestic and commercial food is likely to occur, and illness has been traced to the ingestion of faecally infected food in multiple outbreaks [9]. The ingestion of infected fresh vegetables and fruits has been related to many outbreaks of human gastro-enteritis [10].The use of untreated waste water and manure as fertilizers for the cultivation of fruit and vegetables is a significant contributor to contamination [11,12].Fresh fruits and vegetables can harbor large and diverse populations of bacteria. However, most of the research on fresh fruits and vegetables has focused on their isolation and, as a result, we know far less about the overall diversity and molecular characteristics of those bacterial communities [13]. We addressed these knowledge gaps by isolating bacteria from different fruits and vegetables and assessing their molecular characteristics through 16 S rRNA sequencing approach.The present study was undertaken to isolate and identify pathogenic bacteria from fresh fruits and vegetables that are very popular in Chittagong region, Bangladesh.
2. Materials and Methods
- Sample collectionA total of 7 samples of fresh fruits and vegetables were collected from two open markets (Baluchara, Masjid Market and Oxygen market) of Chittagong city. The samples were Cucumber (Cucumis sativus), Carrot (Daucus carota), Tomato (Solanum lycopersicum), Hog plum (Spondias mombin), Guava (Psidium guajava), Apple (Malus domestica) and Jujube (Ziziphus). All samples were collected in a sterile polythene bag in an insulated ice box to maintain temperatures ranging from 4° to 6°C and analyzed within one hour of receipt. Preparation of samplesWithin an hour of collection, the samples were taken into the laboratory and rinsed for each with 100 ml of distilled water, then diluted 10 fold serial. After washing the vegetables surface 10 ml of washed aqueous suspension of each sample was mixed with 90 ml Luria Bertani (LB) broth and incubated for 24 h at 37°C. This overnight culture in LB broth was used for the isolation and identification of strains on selective media in streak plate technique [14,15].Isolation and identification of bacteriaThe bacteria were isolated and enumerated by growing them on selective and non-selective mediums such as nutrient agar was used for total viable bacterial count (TVBC) and for total coliform (TC) and fecal coliform (FC) count MacConkey broth was used. One loop full culture from LB broth was streaked over selective media and kept it for incubation overnight at 37°C.Eosin methylene blue (EMB) for E. coli and thiosulfate citrate bile sucrose agar (TCBS Agar) for Vibrios Spp., Vibrio cholera like organism (VCLO), xylose lysine deoxycholate agar (XLD agar) and salmonella-shigella sgar (S-S Agar) for Salmonella, tomato juice agar for Lactobacillus spp. Cetrimide agar for Pseudomonas spp. and mannitol salt phenol-red agar was used for Staphylococcus spp. For identification confirmation IMViC tests were done for every species [16].Bacterial enumerationSpread plate method was used for bacterial enumeration to determine the number of colony forming units (CFUs) [17].Statistical AnalysisFor statistical analysis, the SPSS program (V12) was used and the T test was performed to determine the significance of the difference between groups. At 95 percent conviction (p<0.05), the significance between the values was assessed [18].
3. Results
- Total Viable Bacterial CountIn this study, all sampled fruits and vegetables were contaminated. The microbial load of fruit and vegetables varied with the types. Range of microbial count of guava was 2.1×104 CFU/ml, apple was 4.3×104 CFU/ml, tomato was 8.2×104 CFU/ml, cucumber was 5.0×104 CFU/ml, carrot was 1.3×104 CFU/ml, hog Ppum (Amra) was 7.5×104 CFU/ml and jujube (Boroi) was 1.2×104.Isolation and identificationA total of 4 bacteria were isolated from each of the samples and they were identified on the basis of the cultural, morphological and biochemical characteristics (Table: 1).
|
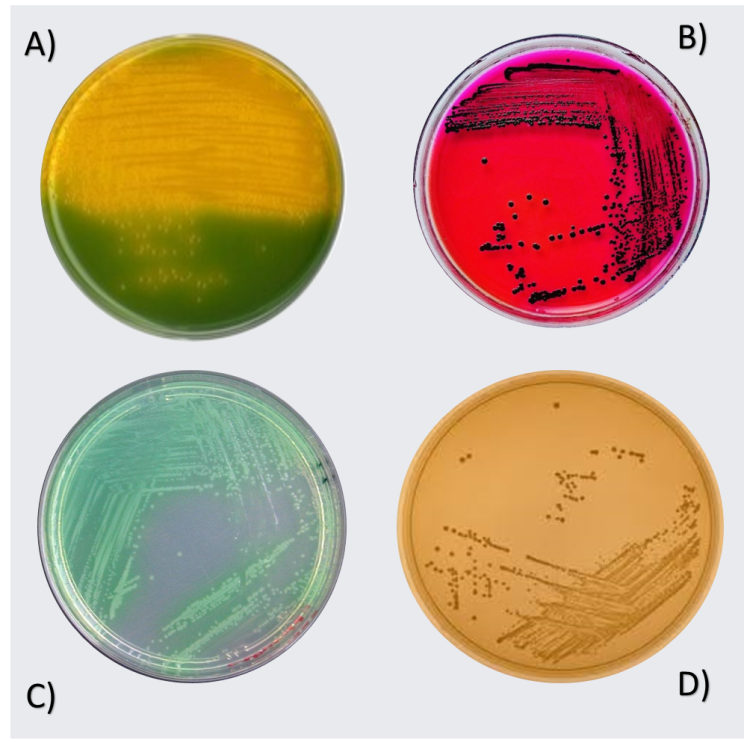 | Figure 1. A) Vibrio spp. B) Salmonella Spp. C) Lactobacillus spp. D) Pseudomonas spp |
4. Discussion
- During cultivation in fields or orchards or during harvesting, post-harvest handling, refining and distribution, vegetables and fruits become infected with pathogenic microorganisms. [19].The presence of all four types of bacteria in the fruits and vegetable samples is probably a reflection of the nature of the two retail outlets in the study [14]. This result highlights the fact that fresh vegetables could be contaminated with pathogenic bacteria and thus could possibly act as a transmission vehicle of many diseases. The prevalence of coliform in guava was 2.1×104 CFU/ml, apple was 4.3×104 CFU/ml, tomato was 8.2×104 CFU/ml, cucumber was 5.0×104 CFU/ml, carrot was 1.3×104 CFU/ml, hog plum (Amra) was 7.5×104 CFU/ml and jujube (Boroi) was 1.2×104 which clearly indicated that the unhygienic condition of open markets.The Pseudomonas spp. was present in almost all the samples where presence of Salmonella spp. was less in among the samples. The detection of Vibrio cholera in particularly 20% of the samples is a matter of concern. Identification of Vibrio spp., Lactobacillus spp., Pseudomonas spp. and Salmonella spp. is a clear sign of serious health crisis alert for of the people living in this area.This was clear that samples from wet markets yielded a higher proportion of bacteria because it could be seen that the way vegetables were treated on the wet markets was less hygienic. The surroundings and places for vegetable displays on markets were not clean and tidy, and the handlers did not wear gloves while the vegetables were being handled. Contamination might be occurred through a contaminated container for transporting and improper handling [20]. Apart from that, the vegetables at supermarkets sometimes have a long holding time, which could contribute to the accumulation of pathogenic bacteria [21].
5. Conclusions
- This study demonstrated the alarming presence of pathogenic bacteria among the most common and popular fresh fruits and vegetable in this area. The result also indicated that the current hygiene condition of selling and buying zone of fresh fruits and vegetable in Chittagong. This study provided a general overview of the microbiological quality of fresh fruits and vegetable in Chittagong which will help to take necessary step to make sure the hygiene condition during fruits and vegetables selling and buying.
ACKNOWLEDGEMENTS
- We would like to show our gratitude to Bangladesh Council of Scientific and Industrial Research, Industrial Microbiology Division.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML