-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2020; 10(2): 45-54
doi:10.5923/j.microbiology.20201002.02

Microbial Quality Spectrum of Packed and Fresh Fruit Juices in Gondar Town Supermarkets and Cafes, Northwestern Ethiopia
Meseret Berhanu1, Mussa Adal2, Samuel Sahile3
1Department of Biology, Applied Microbiology, Mekedela Amba University, Ethiopia
2Department of Biology and Biotechnology, Wollo University, Ethiopia
3Department of Biology, Applied Microbiology and Plant Pathology, University of Gondar, Ethiopia
Correspondence to: Meseret Berhanu, Department of Biology, Applied Microbiology, Mekedela Amba University, Ethiopia.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Packed and fresh fruit juices are important parts of the diet of all age groups due to associated health benefits. However, these food sources are highly perishable and affected by different microbial contaminants from the processes of production to consumption. In this regard, an assessment of the microbial quality spectrum of packed and fresh fruit juices was done. The objective was to evaluate the status of microbe free food handling of the cafes and super markets. Cross sectional study was used as a study design. A total of 48 fruit juice samples were analyzed for Aerobic Mesophilic Count, total coliform, mold and yeast count and pathogen detection. The data was analyzed using one way ANOVA by spss version 20. The results revealed that mean values for Aerobic Mesophilic Count ranged from 7.24 X 103 to 2.68 X 103 cfu/ml for packed and 5.2 X 104 to 4.5 X 103 cfu /ml for fresh fruit juice. The total coliform count ranged from 3.89 X 103 to 2.55 X 103 cfu/ml for packed and 1.76 X 104 to 1.36 X 103 cfu/ml for fresh juice. Mold and yeast count ranged from 1.35 X 102 to 1.78 X 102 cfu/ml for packed and 2.4 X 102 to 3.4 X 102 cfu/ml for fresh juice. Two genera of molds (Alternaria spp. and Fusarum spp.) were isolated from 60 samples. Statistically significant difference (P=0.021 < 0.05) was recorded between Rani and Apple on Aerobic Mesophilic Count. Similarly, significant difference (p=0.002 < 0.05) was recorded between Rani Mango and Pine apple for total coliform counts. A highly significant mean difference (p=0.0 < 0.05) was obtained for total yeast and mould counts. The highest detected species was Staphylococcus aureus (16, 26.6%) from fresh and packed juice samples whereas, the least was Salmonella spp. (5, 8.3%) from fresh juice samples. Escherichia coli and Salmonella spp. were absent in all packed fruit juice samples. All the pathogens were sensitive to gentamicin but resistant to amoxicillin. The microbial quality spectrum of packed fruit juice was better than that of fresh juice samples collected from local cafe.
Keywords: Fruit juices, Microbiological spectrum, Microorganisms, Pathogens, Spoilage
Cite this paper: Meseret Berhanu, Mussa Adal, Samuel Sahile, Microbial Quality Spectrum of Packed and Fresh Fruit Juices in Gondar Town Supermarkets and Cafes, Northwestern Ethiopia, Journal of Microbiology Research, Vol. 10 No. 2, 2020, pp. 45-54. doi: 10.5923/j.microbiology.20201002.02.
Article Outline
1. Background
- Fruit juices are beverages that are important parts of modern diet in many countries of the world. The various forms of fruit juices including whole fruit, fruit juice, fruit pulp, and fruit concentrate are dietary sources of nutrients for humans which are essential for health. The commonly grown fruit types in Ethiopia include apples, asparagus, avocado, banana, citrus fruits, guava, grapes, mandarin, mangoes, papayas, passion fruits, pineapples and oranges. Fruit juices help prevent various deficiency diseases through supplying mineral and vitamin. Their incorporation in the diet and consumption is vital in maintaining a healthy body weight [9] and reduce risk of several diseases [1,2]. It was reported that fruits contain vitamin C, folate, dietary fibers and other bioactive compounds including carotenoids and flavonoids and their low intake is estimated to cause about 31% of heart disease and 11% of stroke worldwide [30]. Moreover, [35] has described that some tropical fruits are known to have therapeutic properties and are popularly used traditional medicines in several countries. Besides the role that fruits play in health aspect, fruit products have become valuable making a substantial contribution to the economy of the international trade market [33]. In Ethiopia, the potential that fruit juices have for domestic and export market is great.Several studies revealed that fruit juices are contaminated with various food borne pathogens including Salmonella, Shigella, Vibrios, Escherichia coli [37], Saccharomyces cerevisiae, Candida lipolytica and Zygosaccharomyces spp. [38]. Similarly, another author [4] reported common cases of food borne infections that arise as a result of fruit contamination by entero-pathogenic bacteria such as Salmonella, Vibrio cholerae, Vibrio parahaemolyticus and Staphylococcus aureus. Poor handling practices at times of harvest, packaging and transporting cause cut and damage on fruits which in turn make the product susceptible to contamination by microorganisms [39]. The contribution to fruit juice contamination by spoilage and pathogenic microorganisms due to improper sanitary and unhygienic conditions associated with vendor, street dusts, environmental conditions and storage places is also very high [2].Poor packaging, handling and preparation process by vendors cause health risk and dissatisfaction of consumers. The contamination of fruits by spoilage and pathogenic microorganisms results not only in human health disorders but also in yield loss and reduction of the national and international market economy. Very little research on fruit microbial quality spectrum assessment was carried out in the study area [2,36]. It is reported that sample fruit juices collected from supermarkets are found available in canned and their extraction and handling is not hygienic and not acceptable for consumption. [2,4]. The study aimed to conduct an assessment of microbiological quality of fruit juices (fresh and packaged) in some supermarkets and cafe of Gonder town and investigates the possible route of contamination.
2. Research Methodology
2.1. Research Setting
- The study was conducted in Ethiopia, Gondar town which is located at 12° 35' 18.74" N to 37° 26' 24" E at elevation of 2080m a. s.l. It is 747 Km away from Addis Ababa (the capital city of Ethiopia) to the North West. Based on the 2007 census, the total population of Gondar is 207,044 (98,120 males and 108,924 females) [8]. It also has a mid altitude climate and annual maximum and minimum temperature of 20°C and 16°C respectively.
2.2. Research Design
- The study design was experimental (interventional study design). It was a cross sectional study and true experimental type. A laboratory- based cross sectional study was conducted for about six months between December 2016 and May 2017 to evaluate the microbiological quality of packed and fresh juices. Twenty four fruit juices were collected from supermarkets and cafés of Gonder town following which microbiological enumeration and identification was done using standard methods.
2.3. Sample and Sampling Techniques
- Samples including Pineapple and Rani Mango were collected both from packed and fresh fruit juices. Representative samples were selected through simple random and stratified sampling technique. Samples were bought from supermarkets and cafes. The samples of the study comprised of both packed and fresh fruit juices particularly none refrigerated ones. Among the total of 48 fruit juice samples collected, 24 were taken from each. The samples were kept at -4°C refrigerator for further analysis.
2.4. Sample Collection
- A total of 48 fruit juice samples, packed and fresh fruit juices 12 each (pine-apple and Rani Mango both packed and fresh) were purchased from super market and cafe. The samples were collected using clean and sterilized container. With regard to assortment of sample for analysis, the expiry date of samples used was checked. The experiment was conducted before the expiry date of samples. Observation was the sound tool for collecting data and data handling practices of packed and fresh fruit juices across supermarket and cafe.
2.5. Sample Preparation for Microbiological Analysis
- Twenty five milliliter (25 ml) of the fruit juices was separately drawn and diluted in 225 ml of sterile physiological saline solution (0.85% NaCl). Fruit juice samples were homogenized using Stomacher [14] at 230 rev/min. One milliliter (1 ml) of each homogenized packaged and fresh fruit juice samples was serially diluted with nine fold sterile peptone water. Sample preparation and microbiological analyses were made side by side for both packed juice and fresh juices. The pH of the samples was measured using digital pH meter after homogenizing 10 ml of the fruit juices in 90 ml of sterile peptone water [2]. Out of the tenfold serially diluted juice samples, three dilutions: 10-3, 10-4 and 10-5 were used for enumeration and isolation of microbes throughout the experiment for all fruit juice samples. Each dilution was inoculated on different bacterial growth media and spread using Spread plated technique.
2.6. Enumeration of Microbes
2.6.1. Aerobic Mesophilic Count (AMC)
- Aerobic mesophilic count was determined by spread plate method on plate count agar. Zero point one milliliter (0.1 ml) taken from each serially diluted sample (10-3 to 10-5) was spread on plate count agar medium in triplicates. The samples were incubated at 37°C for 24 hours. After incubation, plates containing colonies ranging from 25-250 were counted. The results were expressed as colony forming units (cfus) [10].
2.6.2. Total Coliform Count
- The total coliform count was determined by multiple tube fermentation technique using the three test tube method in which one milliliter (1ml) of sample was diluted up to a factor of 10-6. An Aliquot dilution was added to lauryl sulphate tryptose broth contained in inverted Durham tubes that were adjusted to show the formation of gas. For presumptive enumeration of coliforms, the test tubes were incubated at 37°C and were examined after 18 to 24h. The formation of sufficient gas that fills the concavity at the top of the Durham tube was considered to be “presumptive positive”. Those that showed positive test were inoculated in Brilliant Green Lactose Bile (BGLB) broth and incubated at 37C for 48 hours. The coliform density was evaluated using most probable number (MPN) [14].
2.6.3. Yeast and Mould Counts
- For Yeast and Mould counts, spread plate method using Sabbauad dextrose agar (SDA) (Don whitely eqp. Pvt. ltd-India) was used. One milliliter (1 ml) of the sample was serially diluted to nine fold sterile peptone water of which zero point one (0.1 ml) taken from each dilution (10-3 to 10-6) of the serially diluted sample was spread on SDA in triplicates. The plates were incubated at 25 ± 1°C for 5-7 days. After incubation, colonies ranging 10 -150 were counted and the results were expressed as colony forming units (cfus) [11].
2.7. Isolation of Bacteria
- Salmonella-Shigella (SS) agar was applied for the isolation of Salmonella and Shigella. Zero point one milliliter (0.1ml) of the sample was spread by spread plate method on the Salmonella-Shigella agar and incubated at 37°C for 18- 24 hours which is the normal incubation time of bacteria. Among the colonies, the colorless colonies with and without black center were transferred to nutrient broth and incubated at 37°C for 24 hours. The cultures were characterized by biochemical tests such as IMViC, H2S and NO3 production and catalase test [5]. Staphylococcus aureus was isolated using Mannitol salt agar (MSA). Zero point one milliliter (0.1 ml) of the sample was spread on MSA and incubated at 37°C for 24-48 hours. Colonies that changed the medium to yellow and others that did not change the color were transferred to nutrient broth and incubated at 37°C. The cultures were further confirmed using tests including coagulase, catalase, MRVP, and indole test [23,2].Isolation of E. coli was done by subculturing all the positive tubes (obtained in MPN tests) onto Eosin Methylene Blue (EMB) agar and incubating them at 37°C for 48 hours. Dark blue-black colonies with a metallic green sheen indicating vigorous fermentation of lactose and acid production that leads to precipitation of a green metallic pigment on the EMBA was isolated as E. coli. Confirmation was done with biochemical test. The presence of Pseudomonas and Klebsiella were determined by spreading 0.1 ml of sample on Macon key agar and incubating at 37°C for 24-48 hours. The cultures were further purified by repeated plating on nutrient agar through incubation at 37°C for 24 hours. Confirmation of the bacterial isolates was done by conducting biochemical test.
2.8. Cultural Characterization of Bacterial Isolates
- The cultural characterization of isolates was done by growing them on selective and differential media including SS agar, Mannitol salt agar, Mac Conkey agar and EMB agar and incubating them at 37°C for 24-48 hours [3]. The E. coli colonies were streaked on EMB agar and differentiated by their characteristic convex, moist and green metallic sheen growth. However, colonies that showed circular, smooth, convex, moist and yellow color after culturing on Mannitol salt agar and incubating at 37°C for 24-48 hours were classified as Staphylococcus aureus [19]. Lactose fermenting bacteria that appeared small pink or red colonies were regarded as E. coli or Klebsiella. Likewise, lactose fermenting bacteria that displayed colorless colonies were considered as Salmonella species, Proteus species, and Shigella species. Production of H2S which turned the center of the colonies black was labeled Salmonella species [17]. Identified and characterized isolates were stored at -4°C refrigerator for further test.
2.9. Biochemical Tests for Bacterial Identification
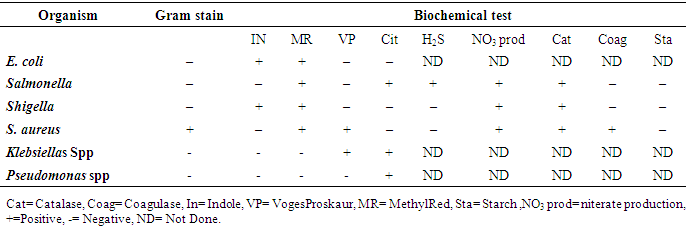
- Identification of isolated bacteria was done using various biochemical tests particularly IMViC tests to differentiate enterics (Family Enterobacteriaceae). Tests included Indole test (tryptone broth), Methyl Red test and Voges-Proskauer tests (MR-VP broth) and Citrate test (Citrate agar slants). IMViC tests were conducted for identification of enteric E. coli while the following tests nitrate reduction test, hydrogen sulfide test, catalase test, urea hydrolysis, starch hydrolysis and coagulase test were used for Enterobactor [18].
2.10. Identification of Yeast and Mould
- Based on macroscopic structures, colonies were sub-cultured and incubated on new SDA agar slant for further characterization. Identification of fungi isolates was done using microscopic methods [15]. Drop of lacto phenol cotton blue stain was placed on a clean slid and a small portion of the mycelium from the fungal cultures was removed and placed in a drop of the stain using mounted needle. The mycelium was spread very well on the slid and a cover slip was gently lowered on it. The slid was examined under the microscope. Observation was done at low and high power objectives of the microscope [20]. Morphological characters of hyphae i.e. asexual reproductive structures were observed and recorded.
2.11. Antibiotics Susceptibility Test
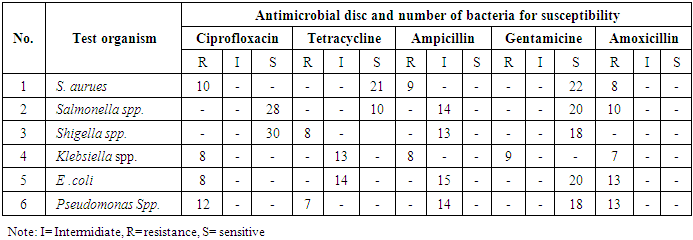
- To determine the antibiotic resistance of bacterial strains, antimicrobial susceptibility test was conducted on Pseudomonas spp., Staphylococcus aureus, Salmonella spp., Shigella spp., E. coli, and Klebsiella spp. by agar disc diffusion standard method using Mueller-Hinton agar [15]. Isolated colony cultures were shifted to normal slain broth to preserve the culture. The culture was vortexed thoroughly and bacterial suspensions were compared to 0.5 McFarland standards. The culture was then swab on the agar disc diffusion medium within 15 minutes and penicillin (10 µg), tetracycline (30 µg), ciprofloxacin (5µg) and gentamycin (10 µg) and amoxicillin (10 µg) discs were applied on the plate individually. The plates were incubated at 35°C for 16 to 18 hours. The diameter of the clear zone was measured and recorded in millimeter. Finally, the result was compared with the zone size standard table and recorded as sensitive, intermediate or resistant to each antimicrobial tested [2,7].
2.12. On Site Observation
- Data about the sanitary conditions, handling and hygienic practices of both packed and fresh fruits were collected on site observation through preparing observation check list (Appendix 1-3).
2.13. Data Analysis
- Quantitative approach was used for data analysis of this study. The data collected were analyzed for ANOVA using SPSS software version 16. Average values were used for triplicate data and all the countable dilution were used to calculate the average number of colonies in terms of colony forming unit per milliliter cfu/ml for Aerobic Mesophilic Count (AMC), Yeast and Mould count. Microbial density was expressed using most probable number per mililiter (MPN/ml). P < 0.05 was taken as statistically significant association (2, 4).
3. Results
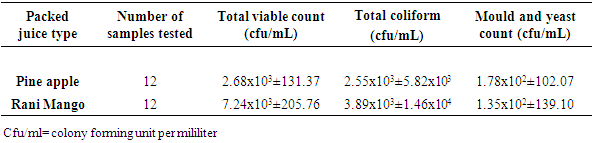
3.1. Microbiological Enumeration from Packed Fruit Juices
- The number of samples and count ranges in cfu/ml of Aerobic mesophilic count, total coliform, Yeast and mold count were presented (Table 1). Mean Aerobic Mesophilic Count of Pineapple and Rani Mango were 2.68x103 and 7.24x103cfu/ml, respectively. The mean total coliform count of Pineapple and Rani Mango were 7.62x102, 2.55x103 and 3.89x103cfu/ml, respectively. The mean Yeast and Mold Count of Pineapple and Rani Mango were 1.78x102 and 1.35x102cfu/ml, respectively. Rani Mango had high Aerobic Mesophilic Count (7.24x103cfu/ml) and Total Coliforms (3.89x103cfu/ml) as compared to other packed juice type. But pineapple had high Yeast and Mold Count (1.78x102cfu/ml) as compared to Rani Mango (1.35x102cfu/ml).
|
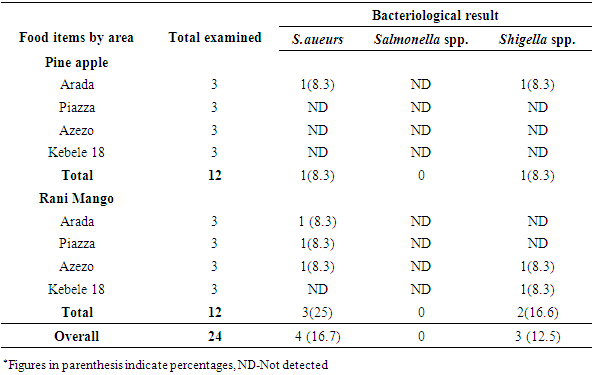
3.1.1. Bacteriological Pathogen Detection from Packed Fruit Juices
- Some packed fruit juice samples were contaminated by more than one pathogen (Table 2). Among packed fruit juice samples, 4(16.7%) were contaminated by Staphylococcus aureus. Consequently, 1(8.3%) Pineapple juice and 3 (25%) Rani Mango were contaminated by Staphylococcus aureus. None of packed fruit juices were infected by Salmonella spp. Two Pine apple fruit juices collected both from Arada area were contaminated by Staphylococcus aures and Shigella spp. Three Rani Mango juices collected each from Arada, Piazza and Azezo were contaminated by Staphylococcus aures whereas two Rani Mango each from Azezo and Kebele 18 were contaminated by Shigella spp. The highest occurrence of Staphylococcus aureus 3 (25%) were recorded in Rani Mango while the least was in Pineapple 1 (8.3%). The result also indicated that 3 (25%) fruit juices were contaminated by Shigella spp. of which 1(8.3%) was for Pineapple and 2 (16.6%) for Rani Mango. The highest occurrence of Shigella spp. (2, 16.6%) was recorded from Rani Mango while the least (1, 8.3%) was from Pineapple.
|
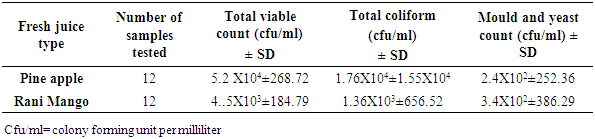
3.2. Microbial Enumeration in Fresh Fruit Juice Samples
- Result of fresh juice type, numbers of samples and count ranges expressed in cfu/ml for Aerobic Mesophilic Count, total coliform, mold and yeast count were presented (Table 3). The Aerobic mesophilic count of Pineapple and Rani Mango were 5.2 X104 and 4.5 X103cfu/ml, respectively. The total coliform count for Pineapple and Rani Mango were 7.62x102 and 1.36X103 cfu/ml respectively. Likewise, the mean yeast and mold count of Pineapple and Rani Mango were 2.4X102 and 3.4X102cfu/ml, respectively. Total viable count, total coliform and mould and yeast count were presented (Table 3). Mean result indicated that fresh juice Pineapple showed high aerobic mesophlic count (5.2 X104) cfu/ml and total coliform (1.76 X 104) cfu/ml compared to value recorded from Rani Mango. However, cfu/ml for Pineapple showed less mean value of total yeast and mold count (2.4X102) cfu/ml as compared to Rani Mango (3.4X102) cfu/ml. Significant difference was recorded for total coliform count (p=0.002 < 0.05), the total count for Yeast and Mould (p=0.468 > 0.05) and aerobic mesphilic count (p=0.469>0.05) was insignificant.
|
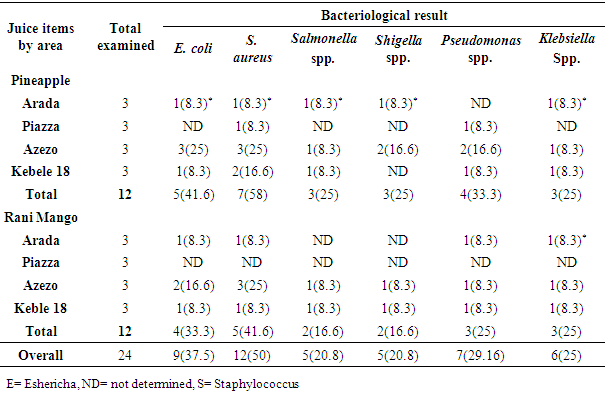
3.2.1. Bacteriological Pathogen Detection from Fresh Fruit Juice
- Occurrence of six different types of bacterial pathogens including E. coli, Staphylococcus aureus, Salmonella spp., Shigella spp., Pseudomonas spp. and Klebsiella spp were detected in Pineapple and Rani Mango fresh fruit juices (Table 4).
|
3.3. Biochemical Results of Bacterial Isolates
- Coliform bacteria were identified through conducting several rapid biochemical tests (Table 5).
|
3.4. Identification of Fungi Isolates
- The macroscopic and microscopic identification tests performed in all fresh juice and packed fruit juice samples indicated two fungi genera, Alternaria and Fusarium.
3.5. Antimicrobial Susceptibility Test of Isolated Pathogens
- The potential of isolated bacterial pathogens to resist selected antibiotics was confirmed by comparing with the control (Table 6). Inhibition zone for the positive control was considered zero (0) mm.
|
3.6. On Site Observation
- Regarding the sanitary conditions, 3 (25%) of selected juice cafes weren’t free from pest, dust, had no collecting bin and toilet room around juice preparation areas (Table 7). Hygienic practices of fruit juice handlers indicated 16.7%, 55.5% and 55.5% didn’t clean nails, didn’t wear clean and appropriate clothes and had no hair nits (Table 8). The handling practices of fruits by workers as observed on site indicated that 33.3% of fruit storage was inappropriate, 100% were stored under unsuitable temperature, 58.3% not packed properly and 91.6% of fruit handlers didn’t wear gloves (Table 9).
4. Discussion
- Based on the findings of the study, high Aerobic Mesophilic Count of packed juices is associated with several factors. The bacterial counts recorded in the study were higher than that of previous reports where 1×105cfu/cm2 [11] was recorded that can be attributed to pre- and post-harvest storage conditions, inappropriate handling during transportation, usage of preservative and pasteurization temperature [2,13,26]. The bacterial count recorded from Rani Mango was higher (7.24×103cfu/ml) than that of Pineapple (2.68x103) juices due to a relatively higher (3.4-5.2) pH of Rani Mango since too low pH in fruit juices inhibits growth of bacteria [29]. Significant differences (p-value < 0.01) were recorded on dependent variable and Aerobic Mesophilic Count (p=0.045, < 0.05). However, no significant differences (p=0.65, 0.21 > 0.05) were observed in Yeast and Mold Count and total coliform count indicating equal contamination of fruit juice samples.Although no specification set is available for acceptable level of microbes in fruit juices being delivered in Ethiopia [4], the AMC and TCC of fresh juice microbial counts of this study has highly exceeded the permitted count of the Gulf standard for total viable count, coliforms, yeast and molds count of 1x104, 1 x 102, and 1x103cfu/ml, respectively [Gulf standards, 2000]. The high counts coupled with isolation of potentially pathogenic bacteria including Shigella and Salmonella spp. cause hazard for health and were unsatisfactory for consumer intake. The microbial count of Pine apple juice in this study was in the level of the permitted count provided that there was no isolation of potential pathogenic bacterial species such as Shigella and Salmonella. Consequently, among fruit juice samples, Pineapple juices were safe and satisfactory to consumers in the study area. However, other packed and fresh juices of the study samples were more contaminated and considered unsatisfactory for consumption.High count of total coliform in multiple tube fermentation test of Rani Mango was recorded over other packed fruit juice types indicates coliform contamination of packed juice could be related to inappropriate processing, use of contaminated water during preparation and washing of fruit or secondary contamination via contact with contaminated equipments [21]. Total Yeast and Mould count of fruits showed a high microbial load a little below the Gulf Standard. Occurrence of high Yeast and Mould counts in Pineapple juices compared to other packed fruit juices could be due to poor handling of fruits, sanitary problem of some marketing areas and poor hygienic conditions during extraction of juices [13]. A statistically significant difference (P=0.0< 0.05) was obtained between fruit juices collected from café and fresh juice samples collected from market places in Aerobic Mesophilic Count and Yeast and Mould count. This implies that fruit juices get more contamination in market places.Aerobic Mesophilic Count from Rani Mango for packed fruit juices and Pineapple for fresh fruit juices being higher than of Rani Mango (fresh) but smaller than the value (8.0 x106) agrees with [29] showing that commercially packed juice is better than fresh fruit juices sold in the café which might be due to effect of automated machine and the preservatives used during fruit juice processing [27]. Absence of E. coli in all packed fruit juice is attributed to quality of potable water used for preparing juice and is in line with [22], absence of Salmonella spp. might be due to minimal use of contaminated animal manure during fruit cultivation.Occurrence of Shigella spp. in packed and fresh juice samples indicates contamination of fruits from poor sanitation although its source remains uncertain [6]. The occurrence of Staphylococcus aureus in packed and fresh juice samples coincided with some previous results [22] which may be due to poor fruit handling by both sellers and buyers (2). Presence of Staphylococcus aureus in fruit juices might be indication of contamination arising from contact with skin, mouth or nose of juice handlers during handling, coughing and sneezing [25]. Salmonella was detected in fresh fruit juice of Pine apple and Rani Mango juice but not in packed juice samples indicating contamination (2). Fresh fruits are being considered as a vehicle of transmission of Salmonella since contamination can occur at several steps along the food chain [6].The highest occurrence of E. coli in fruit juice samples of Pineapple may be attributed to processing failure or post-processing contamination particularly of fecal contamination which is the usual case that occurrence of E. coli in juice is an indication of poor hygienic practice of food handlers [12] that agreed with the finding that [2] has reported. High microbial load and pathogen detection in Pine apples compared to other fruit juices may be due to the suitability of Pineapple for good microbial growth and survival at a relatively moderate pH. Fusarium and Alternaria species of fungi identified from both packed and fresh fruit juices are known to cause fruit spoilage that in turn contribute to post-harvest loss and generating toxic secondary metabolites resulting into a health risk on the consumer [24].Pathogens isolated from both packed and fresh juices in this study showed variable sensitivity and resistance patterns to the tested antibiotics. Pseudomonas spp. resisted all tested antibiotics with intermediate response to ampicillin. Pathogens of this type having multiple drug resistance are extremely serious public health problems and have always been associated with outbreak of major epidemics throughout the world [20]. Relatively fewer bacteria or tested organisms found resistant to ciprofloxacin than to amoxicillin in this study indicating least effectiveness of antibiotics towards pathogens. More sensitivity of tested organisms to gentamicin indicates the effectiveness of this antibiotic as a drug for treating infections arising from eating fruits.
5. Conclusions
- Based on the findings of this study, the total viable count, total coliform and total mould and yeast count showed highest microbial load from fresh Rani Mango fruit juice samples. The microbial detection result indicated that packed fruit juices of Pineapple and Rani Mango were contaminated by three pathogenic species including Staphylococcus aureus., Salmonella species and Shigella species. Analysis also indicated that six pathogenic species E. coli, Staphylococcus aureus, Salmonella spp., Shigela spp., Pseudomonas spp. and Klebsiella spp. were detected in fresh fruit juices of Pine apple and Rani Mango. Staphylococcus aureus was source of contamination in packed fruit juice samples, Staphylococcus aureus and E. coli in fresh fruit juices. Microbial detection result confirmed Staphylococcus aureus, Salmonella species and Shigella species are having wide contamination coverage in both packed and fresh juice samples.Commercially packed fruit juice was less contaminated by pathogenic microbes than fresh fruit juices sold in the café which might be due to effects of automated machine and preservatives used during fruit juice processing. Similarly, fresh fruit juices in this study were contaminated with high microbial load than packed fruit juices due to the poor sanitary conditions, lack of experience of pasteurization, poor hygienic and handling practices. Moreover, total viable and total coliform count of Pineapple was higher than other fruits due to relatively higher pH of Pineapple (5.4 - 5.6) that favors the growth of bacteria. The pH value of Pineapple in this study is close to optimum pH range and as a result more coliform count was recorded in Pineapple than in other fruits recorded at pH value 2.8- 4.9. High bacteria counts coupled with isolation of potential pathogenic bacteria including Shigella and Salmonella spp. is a hazard for health and fitness for consumer intake. The microbial count of Pineapple juice in this study was in the level of the permitted count provided that there were no isolation of potential pathogenic bacteria species such as Shigella and Salmonella. Consequently, Pineapple juices of the study area were safe and fit for consumption. However, other packed and fresh juices in the study samples were unfit for consumption. Absence of E. coli in all packed fruit juice is ascribed to quality of potable water used during juice preparation.Pathogens isolated both from packed and fresh juice showed variations in sensitivity and resistance patterns to tested antibiotics. Resistance of tested organisms to ciprofloxacin and amoxicillin indicates that these organisms could be harmful to human health. Klebsiella resistance to antibiotics except tetracycline, Staphylococcus aurues and Pseudomonas spp. to appreciably more tested antibiotics implies that the organisms may pose hazards to human health. Higher sensitivety of tested organisms to gentamicin and decreasing levels Staphylococcus aurues, Shigella spp. and Salmonella spp. to antibiotics points that these antibiotics can be consulted for remedy of health problems originating from contaminated fruit juice consumption.
Abbreviations
- AMC: Aerobic mesophlic count; ANOVA: Analysis of variance; BGLB: Brilliant Green Lactose Bile; Cfu’s: Colony forming units; Cfu/ml: Colony forming unites per milliliter; EMBA: Eosin Methylene Blue Agar; E.coli: Escherichia coli; H2S: Hydrogen sulfide; IMViC: Indole, Methyl Red and Voges-Proskauer and Citrate test; Ml: Mliliter; MPN: Most probable number; MR-VP: Methyl Red and Voges-Proskauer; MSA: mannitol salt agar; NaCl: Sodium chloride; NO3: Nitrate; Rev/min: Revolution per minutes; SD: Means and standard deviation; SDA: Sabbauad dextrose agar; SPSS: Statistical package for social science; SS: Salmonella-Shigella; TCC: Total coliform count; YMC: Yeast and mold count.
Declaration
- Availability of data and materialsAll data generated or analyzed in this study are included in this manuscript and additional files.FundingGondar University contributed for all the necessary funds. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
ACKNOWLEDGEMENTS
- This work was supported by funds from the University of Gonder.
Other Data
- 3.7. Observation Result for Assessing the Handling Practices of Fresh Fruit Juice in Cafe3.7.1. Sanitary Condition of 12 Selected Café in Gondar Town
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML