-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2020; 10(1): 11-21
doi:10.5923/j.microbiology.20201001.03

Chemical Composition, Antimicrobial Study Against Human and Plant Pathogenic Microorganisms and Optimization of Bioactive Metabolites Produced by the New Strain Aspergillus oryzae 18HG80 Isolated from Saline Soil (El-Baida Marsh, Algeria)
H. S. Nacef1, R. Belhattab2, L. Larous1
1Department of Microbiology, Laboratory of Applied Microbiology, Faculty of Nature and Life sciences, University of Setif 1, Setif Algeria
2Department of Biochemistry, Laboratory of Applied Microbiology, Faculty of Nature and Life sciences, University of Setif 1, Setif, Algeria
Correspondence to: H. S. Nacef, Department of Microbiology, Laboratory of Applied Microbiology, Faculty of Nature and Life sciences, University of Setif 1, Setif Algeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Antibacterial and antifungal activities of the new strain Aspergillus oryzae 18HG80 isolated from saline soil were studied. Among three isolates of A. oryzae S1, S2, S3, the S1 (Aspergillus oryzae 18HG80) had the most antibacterial activity against Gram -positive and Gram-negative and resistant bacteria (MICs between 7.28 and 21.85 μg mL - 1). A. oryzae 18HG80 was also tested for antifungal activity against indicator fungal phytopathogens Aspergillus flavus, Fusarium oxysporum and Alternaria solani, by dual culture method. The results showed that A. oryzae 18HG80 inhibited their growth with low to moderate activity 9.3% for A. solani, 46% for A. flavus and 37% for F. oxysporum. The effects of culture conditions on antimicrobial activity were investigated, the fungus exhibited higher antimicrobial activity at 30°C, pH 6, and 28 days of incubation. The addition of NaCl (from 0.5M to 2M) to the medium decreased the activity and showed optimum inhibitory effects on Gram+ bacteria at 1M. Metabolite profiles of the fungus revealed by gas chromatography mass spectrometry GC/MS, allowed to identify 104 bioactive compounds, where butanedioic (succinic) acid and kojic acid were the major compounds with total percentage of 42,284% and 24,845% respectively, which are a useful platform chemicals that can be converted into many other valuable chemicals. They have many applications in agriculture, pharmaceutical activities, food, cosmetic and health care industries.
Keywords: Aspergillus oryzae, Antimicrobial activity, Secondary metabolites, Gas Chromatography mass spectrometry (GC-MS), Fungal extraction, Optimization
Cite this paper: H. S. Nacef, R. Belhattab, L. Larous, Chemical Composition, Antimicrobial Study Against Human and Plant Pathogenic Microorganisms and Optimization of Bioactive Metabolites Produced by the New Strain Aspergillus oryzae 18HG80 Isolated from Saline Soil (El-Baida Marsh, Algeria), Journal of Microbiology Research, Vol. 10 No. 1, 2020, pp. 11-21. doi: 10.5923/j.microbiology.20201001.03.
Article Outline
1. Introduction
- Saline soils occupy about 7% of the land area, they are characterized by the physical ill-suited characteristics for the growth of organisms, but research showed that some microorganisms, especially fungi are able to grow and adapt to such conditions, as this ability is due to the development of a different physiological mechanisms of adaptation [1]. Microorganisms have been used as a source for the production of many bioactive compounds; these include bacteria, algae, fungi and Actinomycetes …etc. Filamentous fungi are widely distributed in the environment, that had the interest of the researchers towards natural products having different biological activities, since that a number of antibiotics have been isolated from different fungi [2], such as Penicillium janczewskii, P. canescens [3]. Fungi are historical important source of secondary metabolites and they continue to provide new chemical entities with novel biological activities [4]. Numerious fungal extracts and/or extracellular products have been found for their antimicrobial activity mainly from the fungus Penicillium [5]. They provide a rich source of compounds for therapeutic applications including antibacterial, antifungal and antiviral agents [2]. Among those fungi Aspergillus oryzae that is considered an important filamentous fungi in Japanese fermentation industry, as well as in commercial enzyme production, on the other hand A. oryzae is insensitive to most available antifungal antibiotics, [6] such as Asperfuran [7]. The aim of this study was first to isolate and molecular identification of a new halotolerant biotechnological important strain Aspergillus oryzae from saline soil (Chott El- Baida, Algeria), than the determination of its antibacterial and antifungal activities against some common pathogenic microorganisms, in addition to the study of the effect of cultural conditions on these activities. Than the identification of the probable bioactive compounds by Gas chromatography–mass spectrometry (GC-MS) technique, these bioactive components represent a novel future studies may allow the development of a medicine and a pharmacologically acceptable antimicrobial agents especially with the halotolerant capacity of the strain.
2. Methods
2.1. Study Area
- El-Baida marsh is one of the natural saline wetlands. It is located between latitudes 5° 53' 20" E - 5° 53' 30" E and latitudes 35° 57' 80" N - 35° 54' 20" N near Hammam El-Sukhna area (Setif region), an area of approximately 12.223 hectares. Soil surrounding the site is characterized by being less salty to salty, alkaline clay with a gradient composition [8].
2.2. Sampling
- The soil samples were taken at a depth of 12 cm in sterile conditions, three soil samples were taken from different areas in the middle of the marsh, and three others from the agricultural area, about 100 meters from the middle of the marsh, The samples were collected and preserved at 4°C.
2.3. Isolation and Identification of Fungi
- Soil fungi were isolated using both methods, direct and dilution, where the medium Potato Dextrose Agar (PDA) was used with a concentration of NaCl as its equivalent in the sample, and PDA free of NaCl as control.• Direct method: 1 g of soil sample was put into a sterile Petri dish, mixed and distributed homogeneously with the medium, then incubated at 28°C until the appearance of colonies. • Dilution Method: 1 g of soil sample was put in 9 mL of sterile distilled water, and attended a dilution series (10-1, 10-2, 10-3, and 10-4). Dishes were incubated a 28°C until the appearance of colonies [9].
2.3.1. Purification and Identification of the Isolated Fungus Aspergillus oryzae
- The fungus was purified on the same medium in which it was isolated repeatedly until a pure colonies were gotten, Afterwards, the identification was carried out according to J.I. and A.D. method [10], Developing colonies were observed after incubation 30°C for 7 days accounting the following characteristics: diameter of the colony, morphological and microscopic characteristics.
2.4. Fermentation and Antimicrobial Assay
- A disc of 6mm of a pure culture, of each studied fungus strains (S1, S2, S3), was cut from 7 days PDA plate, and placed into 250mL Erlenmeyer flask containing 100mL of YES medium (15% (w/v) sucrose, 2% yeast extract, 0.05% MgSO4, 0.1% metal trace (ZnSO4,7H2O 1%, CuSO4,7H2O 0.5%)). The flasks were then incubated at 30°C for 7 days. At the end of the fermentation, the mycelium with the medium was frozen at -20°C for 2-3 hours, after that, frozen mycelial was crushed and centrifuged at 15000 rpm / 15min [11]. Finally, samples were extracted twice with equal volumes of ethyl acetate. The organic layers were evaporated to dryness in a rotary evaporator at 40±1°C, the dry crude extracts were then dissolved in DMSO 50% and stored at 4°C until used [12,13].
2.4.1. Antimicrobial Activity Evaluation Using Disc Diffusion Method
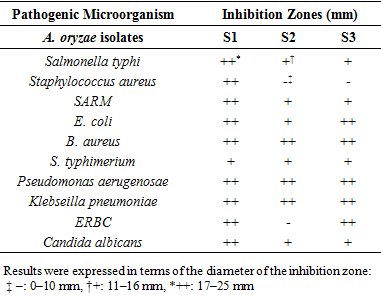
- The ethyl acetate extracts of A. oryzae strains (S1, S2, S3) were individually tested against a panel of microorganisms including a total of 10 microbial cul¬tures, 3 Gram positive bacteria, (Bcillus aureus ATCC10876, Staphylococcus aureus ATCC25923, Staphylococcus aureus resistant Methicillin (SARM) ATCC43300, ), 6 Gram negative bacteria (Salmonella typhi ATCC14028, Escherichia coli ATCC25922, Salmonella typhimirium ATCC13311, Pseudomonas aeruginosa ATCC27853, Klebsiella pneumonia ATCC700603, Escherichia coli resistant a Bactericide ATCC35218.), and the yeast Candida albicans. Muller Hinton Agar plates were prepared by spreading bacteria suspension 108 CFU/mL and Sabouraud for C. albicans with 106 CFU/mL. After that discs of 6 mm diameter were put with 20 μl of the fungal extracts and placed on the inoculated agar. The plates were then incubated at 37°C, 24 h for clinical bacterial isolates and 48-72 h for the yeast [11]. Negative controls were prepared using the same solvents employed to dissolve the extracts. Antimicrobial activity was evaluated by measuring the zone of inhibition developed round the discs. Each assay in this experiment was repeated three times.Antimicrobial activity was considered beyond a diameter of 6 mm or more, and was classified as follows:(–): 0–10 mm; (+): 11–16 mm; (++): 17–25mm [14].
2.4.2. Antifungal Activity
- The antifungal activity of A. oryzae S1 was tested in vitro by the dual culture plate method [15] with modifications, on three fungal phytopathogen indicators, Aspergillus flavus, Alternaria solani, and Fusarium oxysporum. A. oryzae and fungal phytopathogens were cultured separately on PDA plate and incubated at 28°C for 5 days. Then a disc of 6 mm diameter of the isolate and fungal phytopathogens were placed on the opposite site of the same PDA plate, at distance of 5 Cm between the fungal pair, and as control only fungal disc of each fungal phytopathogen was placed at one site of the PDA plate. The plates were incubated at 28°C for 3-5 days. The fungal growth was determined by the radiant of fungal phytopathogen and the percentage of inhibition was calculated by the following formula:
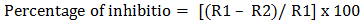 Where: R1 is the average diameter of the fungus growth in control plate; R2 is the average diameter of the fungus growth in test sample plate (Fig. 1).The percentage of inhibition was categorized on growth inhibition category level from low to very high antifungal activity: < 30% = low; 30-50% = moderate; 50-70% = high; ≥ 70% = very high.
Where: R1 is the average diameter of the fungus growth in control plate; R2 is the average diameter of the fungus growth in test sample plate (Fig. 1).The percentage of inhibition was categorized on growth inhibition category level from low to very high antifungal activity: < 30% = low; 30-50% = moderate; 50-70% = high; ≥ 70% = very high.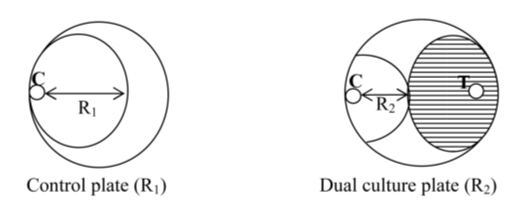 | Figure 1. Measurement of radial growth of pathogens fungi mycelia by dual culture plate method [15] |
2.5. Molecular Identification of the Isolate A. oryzae S1
- Molecular identification of the fungus was carried out in the Laboratory of Microbiology at Neuchâtel University, Switzerland.
2.5.1. Genomic DNA extraction and PCR amplification
- DNA extraction was performed using the kit Quick-DNA™ Fungal/Bacterial Kit de Zymo Research. Then the extracted DNA was quantified via a Qubit® 2.0 (Life Technologies) fluorimeter using the kit. Quant-iT dsDNA BR following the manufacturer's instructions. DNA concentrations of the sample was 39.3 ng / microL. The sample was then diluted to a concentration of two ng DNA / microL for PCR. PCR amplification was performed on the undiluted sample and diluted with Tag enzyme.DNA Polymerase with NEB Thermopol Buffer (New England Biolabs) using a pair of universal primers ITS-1 (5’TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’TCCTCCGCTTATTGATATGC-3’), PCR reactions will be consists of an initial denaturation at 95°C for 5 min, 30 cycles of amplification, and a final extension at 68°C for 5min; each cycle of amplification will be consisted of denaturation at 95°C for 30 s, annealing for 30 s at 55°C, and extension at 68°C for 1 min. Amplification products was electrophoreses in 0.8% agarose gel, stained with ethidium bromide and amplicons will be observed under UV light. After PCR the sample with concentration 38.1 ng / μL was purified by microplate filtration and sent to sequence at GATCBiotech.
2.6. Statistical Study
- To compare the average inhibition zones of the extracts between the three strains of A. oryzae, statistical analysis was occurred using SAS/STAT ® 9.2. The results of the antimicrobial activity were analyzed statically by the two way ANOVA. Statistically significant effects were determined using T test at p <0.05.
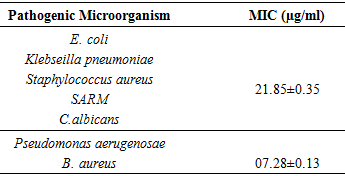
2.7. Determination of the Minimum Inhibitory Concentration (MIC)
- In general, MIC is the lowest concentration that inhibits visible growth after time incubation period of 18-24hours; here, its determination was made from the measurement of the turbidity induced by the growth of germs studied. Therefore MIC is the smallest concentration for which there is no turbidity [16]. The MIC (mg/mL) of the crude extract of S1 strain was determined using dilution method ELISA plate, where a series of two-fold dilutions (dissolved in DMSO up to 5% final DMSO concentration), were prepared in a 96 well-sterile microplate.100 μl of corresponding media MHB for the bacteria and PDB for the yeast) were added, then 100 µl of the dilution extract was introduced in each well. These dilutions were inoculated with 25µl of a solution containing 6x107 Colony Formatting Unit per mL (CFU/mL) of bacterial suspension (Bcillus aureus ATCC10876, S. aureus ATCC25923, SARM ATCC43300, E. coli ATCC25922, P. aeruginosa ATCC27853, K. pneumonia, ATCC700603) and yeast suspension Candida albicans of 7x107 CFU/ml [11,17]. The well-used as a negative control was prepared using the inoculum alone. The microplate was incubated at 37°C for 24 hour. The data was the mean of three replicates [14].
2.8. Optimization of Cultural Conditions Affecting Bioactive Metabolites Production
2.8.1. Effect of Temperature
- The effect of temperature on the bioactive compounds production was tested using liquid cultures of Yeast Extract Broth, 100mL of liquid medium was poured into a 250mL conical flask under aseptic conditions, then inoculated with 1mL of fungus spores (6.4x106 spore/mL), and incubated into different temperature ranges (25 to 35°C) for 7 days. After incubation the antimicrobial activity was recorded according to Chaudhary and Tripathy; Mathan [11,18].
2.8.2. Effect of pH
- The fungus was subjected to different pH ranges (4, 6, 8, and 11), to study the optimum pH required for bioactive metabolites yield. 100mL of liquid medium was poured into a 250 mL conical flask under aseptic conditions, then inoculated with 1mL of fungus spores (6.4x106 spores/mL), and incubated at 30°C for 7 days, After incubation the antimicrobial activity was recorded according to Chaudhary and Tripathy; Mathan [11,18].
2.8.3. Effect of Salinity
- To study the effect of different concentrations of NaCl on bioactive compounds production, the fungus was incubated in various concentrations of NaCl (0.5M to 2M), while other parameters were kept at optimum level. The bioactive metabolite production for each sodium chloride concentration was evaluated [18].
2.8.4. Effect of Incubation Time
- With keeping other parameters at optimum level, the fungus was incubated for (7 days, 14 days, 21 days and month), then bioactive metabolites production corresponding for each time of incubation was estimated.
2.9. Identification of Bioactive Molecules by Gas Chromatography Mass Spectrometry (GC-MS) Analysis
- GC-MS analysis was carried out to identify bioactive compounds of the extract based on the growing of the studied fungus on YESB medium. The same culture medium without fungus was used as control.GC-MS analysis of S1 extract was carried at the Biotechnological Research Centre Laboratory by using column DB-5 (30m x 0.25mm x 1μm) composed of 100% Dimethylpolysiloxane. Carrier gas Helium (99.999%) with constant flow rate of 1mL/min, kg was injected into the program GC- MS instrument. Oven temperature programed: 110°C- 2 min hold, up to 200°C at the rate of 10 °C/min-No hold, up to 280°C at the rate of 5°C/min- 9 min hold, Injector temperature 250°C, Total GC running time 36 min. MS Program: MS were taken at 70eV; scan interval 0.5 seconds and fragments from 45 to 450 Da. Total MS running time is 36 min. Identification of bioactive constituents: Interpretation on Mass-Spectrum GC/MS was carried out using the database of Biotechnological Research Centre (CRBt) (Database/NIST11.L, Database/W9N11.L, Database/NIST11.L) [11].
3. Results
3.1. A. oryzae Identification
- The morphological characteristics on PDA medium, illustrated that the genus Aspergillus isolated is A. oryzae, where the fungus showed dense colonies with rich white sclerotia (Fig. 2, Fig. 3).
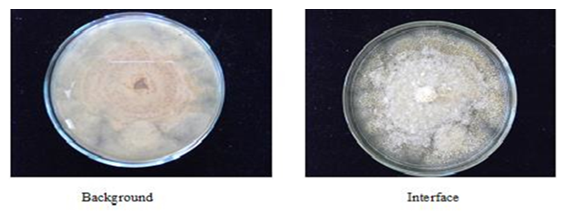 | Figure 2. A. oryzae colony grown on PDA medium at 30°C |
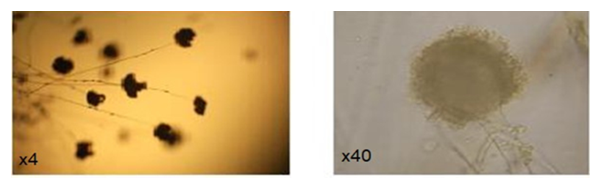 | Figure 3. Soreheads of A.oryzae under the microscope |
3.2. Antimicrobial Assay
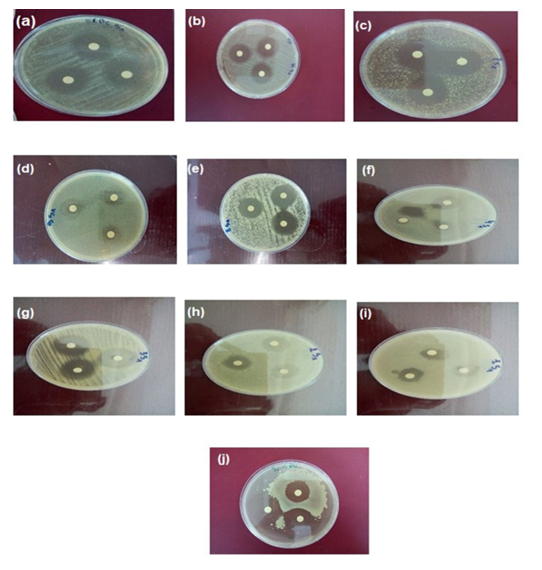
- The three isolates of A. oryzae S1, S2, S3 were tested for their antimicrobial activity, by disc diffusion method; the results showed that there was a variety in the activity with inhibition zone diameters, the isolate S1 demonstrated the highest activity with the mean 13.85 mm, whereas it was 9.11mm and 9.59 mm for S2, S3 respectively. Concerning the effect of the extracts on the pathogenic microorganisms, the extract from S1 showed a bigger zone diameter of 26 mm against P. aerugenosae, whereas these diameters were 23 for S2, and 22 mm for S3 against E. coli. While there was no effect on the bacteria S. aureus by S2 and S3 isolates, in contrast of S1 with 19.4 mm, also for the bacteria ERBC S2 had no effect comparing with the other isolates. As for the yeast C. allbicans the three isolates had a clear effect especially for S1 with 17 mm (Table 1; Fig. 4; Fig. 5). Depending on the statistical results, S1 extract was considered as a very active strain in this assay and was evaluated for the determination of MIC and antifungal activity (Table 2).
|
|
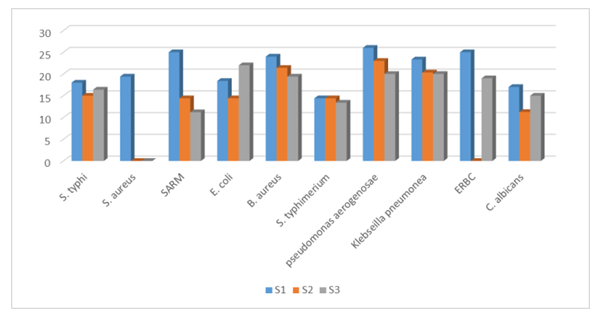 | Figure 4. Comparison of average inhibitions of A. oryzae ethyl acetate extracts on the growth of different groups of pathogenic microorganisms. |
3.3. Molecular Identification of the Isolate A. oryzae S1
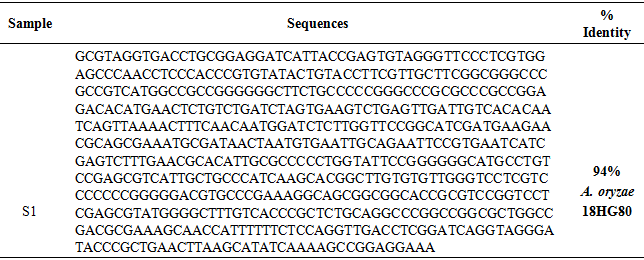
- The PCR purified product and amplified showed in Figure 6, and the nucleotide sequences was illustrated in Table 3, confirmed that the isolated fungus was A. oryzae 18HG80 with 94%.
|
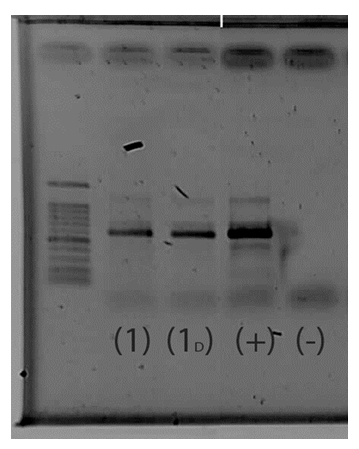 | Figure 6. Gel purified PCR product of Aspergillus oryzae S1 strain (1) A. oryzae S1 Undiluted; (1D) A. oryzae diluted to 2ng / μL; (+) positive control and (-) negative control |
3.4. Antifungal Activity
- Distinct inhibition zones were observed, The fungus inhibited growth of tested pathogens fungi with low to moderate antifungal activity, The percentage inhibition activity against fungal phytopathogens indicators were as follows: A. flavus (46%), F. oxyspouum (37%), A. solani (9.3%) (Fig. 7).
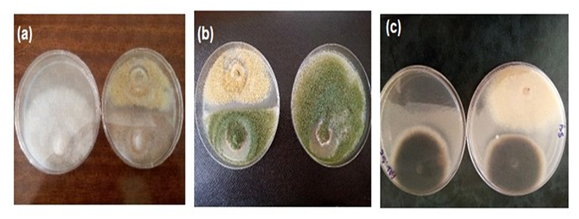 | Figure 7. Antifungal activity of A. oryzae 18HG80 against fungal phytopathogens a- F. oxysporum b- A. flavus c- A. solani |
3.5. Optimization of Cultural Conditions Affecting Bioactive Metabolites Production
3.5.1. Effect of Temperature
- Aspergillus oryzae 18HG80 strain, showed a narrow range of incubation temperatures for effective antibiotic production (Fig. 8). The increase of the incubation temperatures from 25 to 35°C enhanced the production of bioactive metabolites. Maximum inhibition zone was recorded at 30°C against the tested microorganisms.
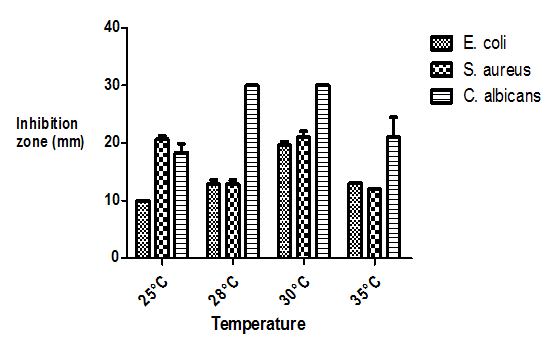 | Figure 8. Effect of temperature on bioactive metabolites production |
3.5.2. Effect of pH
- The antagonistic activity of the test fungus was also influenced by pH of the medium (Fig. 9). The highest antimicrobial activities against three tested microorganisms were obtained at an initial pH 6 of the culture medium; however, at pH 8 and 11 there was no zone of inhibition.
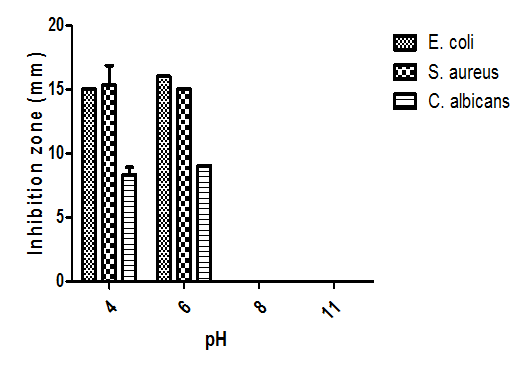 | Figure 9. Effect of pH on bioactive metabolites production |
3.5.3. Effect of Salinity
- The increase of NaCl concentrations from 0.5M to 2M showed that salinity had no significant effect, where antimicrobial activity was observed against S. aureus and E. coli at 0.5M and only against S. aureus at 1M, while there was no effect against C. albicans (Fig. 10).
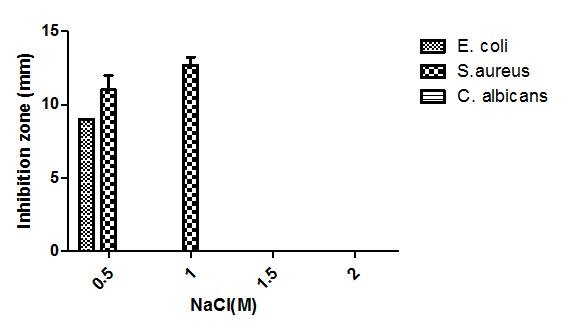 | Figure 10. Effect of salinity on bioactive metabolites production |
3.5.4. Effect of Incubation Period
- Concerning incubation period, antimicrobial activities appeared to be pronounced after the 7th day of growth, this activity remained stable between 7-15 days and then increased at 21th day with a maximum at 28th day of incubation (Fig.11).
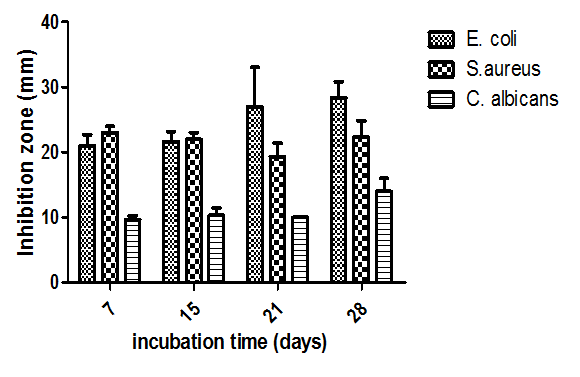 | Figure 11. Effect of incubation period (days) on bioactive metabolites production |
3.6. Identification of bioactive molecules by GC-MS
- The GC-MS chromatograph of ethyl acetate extract was illustrated in Figure 12. There are several peaks in the GC-MS profile. Compounds identified in the extract were 104, the major ones were butanedioic acid (succinic acid) with 42.284% and kojic acid (4H-Pyran-4-one,2,3 dihydro-3,5-dihydroxy-6-methyl-) with 24.845% (Table 4).
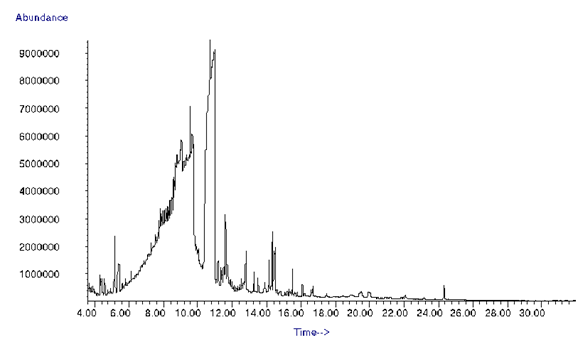 | Figure 12. GC-MS analysis of Aspergillus oryzae 18HG80 ethyl acetate Extract |
|
4. Discussion
- One of the strategies followed in the search for new antibiotic molecules is to explore ecosystems little or not studied, essentially, the ecosystems with one or more factors are extreme (salinity, pH, etc.). The marsh (very salty lakes) which constitute rare regions in the world seem to be promoter environments to isolate fungi source of new antibiotic molecules [19]. Fungal derived natural products have primarily served as lead structures for the development of antibacterial and antiviral agents [20]. In the present study antimicrobial activity was observed for the three isolates’ extracts of A. oryzae isolated from El Baida marsh, because of the secretion of secondary metabolites which have antimicrobial specify [21], same results obtained by Beharka and Nagaraja [22] using direct-fed microbial feed additive A oryzae, they found that bacteria had no positive growth response. In contrast with few scientists [23], those who consider that the fungus A. oryzae is derived from A. flavus family not producing aflatoxins, while Seshime [24] said that A. oryzae contains the gene responsible for the production of aflatoxins, but it is inactive because of mutations in some regulatory genes, same results obtained by Sosa [25] where they found that the fungus didn’t produce mycotoxins as aflatoxins B1,B2, G1,G2 and ochratoxin, while cyclopiazonic acid was detected, thus they concluded that A. oryzae is a safe fungus and could be used as a feed additive for ruminants. In the first time, antibiotics produced by Aspergilli were called aspergillin [21], but recent studies reach that A. oryzae is able to produce many of secondary metabolites as antibiotics such as aspergillormarasmine, cyclopiazonic acid (CPA), kojic acid, 3-nitropropionic acid, maltoryzine and violacetine which was only once reported to be produced on malt extracts as antibiotic. The compound is also produced by Streptomyces [21], in addition to asperfuran as antifungal agent [7]. According to GC-MS analysis the major compound was kojic acid in addition to traces of naphthoquinone and both of them have antibiotic characteristic [14]. Concerning the optimization study, different studies proved that temperature is one of the major conditions affecting the antimicrobial activity. Antimicrobial activity of the fungus increased with temperature from 25- 30° then decreased at 35°, while Miao [26] obtained that antimicrobial activity decreased at 30° and totally inhibited at 35°. Jain and Pundir [27] also obtained maximum production of antimicrobial metabolite by Aspergillus terreus at 25°C.Influence of pH on the production of bioactive metabolites by fungi was well investigated, optimum antimicrobial metabolites production was recorded maximum at pH 6, similar observations were also made by Bhattacharyya and Jha [28], they pointed out that most of the microorganisms have the ability to synthesize antimicrobial compounds at pH ranging from 5.5 to 8.5. Maximum production of antibacterial metabolites by Aspergillus terreus in Potato dextrose broth medium at pH 6.0 was obtained by Jain and Pundir [27] NaCl concentrations 0.5M and 1M were optimum for the production of bioactive metabolites that affect Gram+ bacteria. Comparing with Gram- ones and C. albicans the presence of salinity in the medium inhibited their growth. Similar results were reported by Bhattacharyya and Jha [28], where they found that bioactive metabolites production was gradually decreased along with increasing salt concentration in the basal medium.Concerning the period incubation, the strain required 28 days for optimum production of antimicrobial metabolites, Effect of incubation period on the production of bioactive compounds (Fumonisin B1) by Fusarium moniliforme was investigated by Alberts [29] they observed that the production of metabolite started after 12 days. While Bhattacharyya and Jha [28] found that Aspergillus strain required 16 days for the production of secondary metabolites.
5. Conclusions
- The fungus extract has an antimicrobial activity; especially the isolate A. oryzae 18HG80 against sensitive and resistant pathogenic bacteria, yeast and fungi. Thus A. oryzae 18HG80 plays an important role (tool) in the search for natural products. It might be also represent an alternative source for the production of therapeutic agents and bioactive metabolites that are not easily obtained by chemical synthesis, and which have a high activity against pathogenic microorganisms. However, to value this work it is necessary to pursue it with purification and application in vivo of these bioactive molecules to reach if this strain has the potential to serve as a biological control or as new pharmacological and biotechnological agents. In addition, Succinic acid is an important platform chemical with various industrial uses as it is a precursor for the production of numerous products, such as 1,4-butanediol, resins, coatings, pigments and many others. Succinic acid is conventionally produced in the petrochemical industry. Succinic acid is traditionally produced from petrochemicals, which is costly and causes some pollution problems, but Biological succinate can reduce the use of non-renewable resource.
ACKNOWLEDGEMENTS
- The authors want to thank the Biotechnological Research Centre (CRBt) for their help with the bioactive molecules identification by GC-MS.We are grateful to Dr Bindschedler S. from Nuchâtel University, Laboratory of Microbiology for her help with molecular identification of the Fungus.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML