-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2019; 9(2): 25-28
doi:10.5923/j.microbiology.20190902.01

Detection of Escherichia coli in Dillion Lake in Southeast Ohio
Mohannad AL-Saghir 1, Shadi Abu-Baker 2
1Department of Biological Sciences, Ohio University, Zanesville, Ohio, USA
2Department of Chemistry, Ohio University, Zanesville, Ohio, USA
Correspondence to: Mohannad AL-Saghir , Department of Biological Sciences, Ohio University, Zanesville, Ohio, USA.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Water quality in Ohio has been a growing concern in recent years. The current project was set to meet the following objects: 1) Use the Colilert test in detecting and quantifying the bacterial contamination of water resources; 2) Raise the public awareness of drinking water pollution and its impact on community and surrounding wildlife; 3) Use the current project as model to be used in the future testing of the water sources cross Ohio. Based on the current results, it is determined that E.coli level are mostly with in the acceptable and legal limits. However, its presence is concerning. These bacteria can cause water turbidity and deplete oxygen inside water which kill water life and damage the ecosystem. Presence of such pathogens in water, warrants more effective treatment of water. Farmers shall use more environment friendly fertilizer on their farming land and utilize better filtration system to get rid of their agricultural waste. The current results show that Colibert test is sensitive, accurate and effective in measuring E.coli in water samples. The current methodology can be used in future testing of water in this area and other areas of Ohio.
Keywords: E.coli, Coliform, Water Quality, Dillion Lake, Southeast Ohio
Cite this paper: Mohannad AL-Saghir , Shadi Abu-Baker , Detection of Escherichia coli in Dillion Lake in Southeast Ohio, Journal of Microbiology Research, Vol. 9 No. 2, 2019, pp. 25-28. doi: 10.5923/j.microbiology.20190902.01.
Article Outline
1. Introduction
- Bacterial quality of drinking water in Ohio has been a raising concern recently. Contaminated water can cause many diseases such as diarrhea, cholera and more. It represents an immediate and acute health risk factor (Rompre et al., 2002).Coliforms are group of bacteria present in large numbers in human intestine and other animals, and are thus found in their fecal wastes ((Rompre et al., 2002). Coliforms are used as an index of the potential presence of pathogens in water sources and environments. The coliform group, specifically Escherichia coli, have been used as an indicator of bacterial water quality since the 19th century. Although Coliforms are routinely found in various natural environments, drinking water is not a natural environment for them. Their presence in drinking water must be considered as a possible threat or indicative of microbiological water quality deterioration. Positive total coliform samples in a treated water which is usually coliform free may indicate treatment ineffectiveness, loss of disinfectant, break through (McFeters et al., 1986), intrusion of contaminated water into the potable water supply (Geldreich et al., 1992; Clark et al., 1996) or regrowth problems (LeChevallier, 1990) in the distribution system and should not be overlooked. Coliform group members are described as: 1) all aerobic and facultative anaerobic, Gram- negative, non-spore-forming, rod-shaped bacteria that ferment lactose with gas and acid formation within 48 h at 35°C; 2) or all aerobic and many facultative anaerobic, Gram-negative, non-spore-forming, rod-shaped bacteria that develop a red colony with a metallic sheen within 24 h at 35°C on an Endo-type medium containing lactose (Adams et al., 1989). In 1914, the U.S. Public Health Service adopted the enumeration of coliforms as a more convenient standard of sanitary significance. Although coliforms were easy to detect, their association with fecal contamination was questionable because some coliforms are found naturally in environmental samples (Venkateswaran et al., 1996). This led to the introduction of the fecal coliforms as an indicator of contamination. Fecal coliform is a subset of total coliforms that grows and ferments lactose at elevated incubation temperature. Currently, detection of coliforms is used as an indicator of sanitary quality of water or as a general indicator of sanitary condition in the food processing environment. Fecal coliforms remain the standard indicator of choice for shellfish and shellfish harvest waters; and E. coli is used to indicate recent fecal contamination or unsanitary processing (Venkateswaran et al., 1996). Colilert test is a commercially available enzyme-substrate liquid-broth medium (IDEXX Laboratories, Inc., Westbrook, Maine) that allows the simultaneous detection of total coliforms and Escherichia coli (E. coli). It is available in the most-probable number (MPN) or the presence/absence (PA) format. The MPN method is facilitated by use of a specially designed disposable incubation tray called the Quanti-Tray®. The PA method can be done using any sterile bottle of the appropriate size. Both methods can be done in the field or laboratory. The Colilert method is approved for use with drinking water. It can be used to monitor all types of water, including water that is high in suspended sediment; it can also be used to monitor sediments. The PA format is suitable for use in drinking-water or ground-water studies, when only a presence or absence result is needed. No special equipment is required to use the PA format. The MPN format can be used with all waters; however, it requires the use of a special machine that seals the incubation tray. The current research is designed to connect and continue the research that was conducted by my colleague Dr. Abu Baker (Abu Baker et al., 2018) where they examined the levels of ammonia and nitrates in Dillon Lake and their effects on the environment. Their results indicated the presence of excess nutrients, but within the legal limits.The current project is set to meet the following objects: 1) Use the Colilert test in detecting and quantifying the bacterial contamination of water resources; 2) Raise the public awareness of drinking water pollution and its impact on community and surrounding wildlife; 3) Use the current project as model to be used in the future testing of the water sources cross Ohio.
2. Materials and Methods
2.1. Water Samples Collection
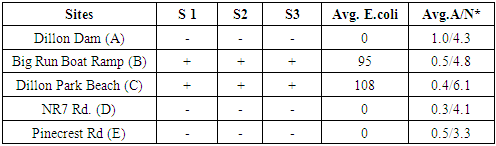
- As in Abu Baker et al., 2018: Five locations were selected around the lake sides to cover most of lake surroundings. The lake area was covered and samples were collected from at least 3 foot depth from the following areas Dillon Dam (A, South side), Big Run Boat Ramp (B, South-east side), Dillon Park Beach (C, East side), North Road 7 (D, North side) and Pinecrect Road (E, West side). Samples locations are shown in Figure 1 and 2 maps (Abu Baker et al., 2018). The samples were obtained in succession and in triplicates. Plastic bottles were sterile and dried.
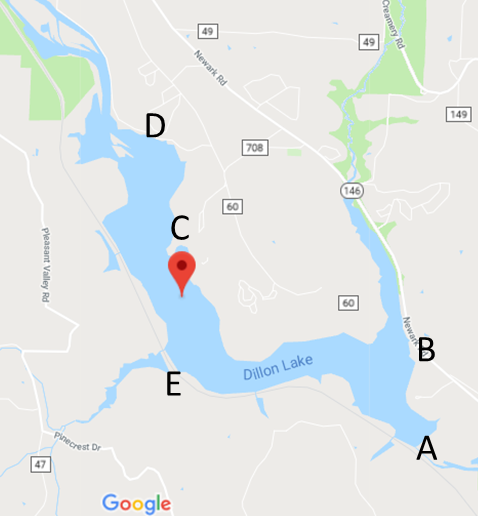 | Figure 1. Map showing the five testing locations. (Map courtesy of Google Maps, inset images by Audrey Lafferty.). Samples’ locations are (A, B, C, D and E) [Abu Baker et al., 2018) |
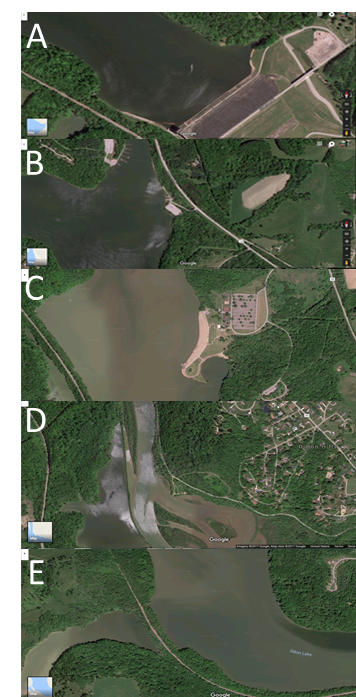 | Figure 2. Satellite Maps showing the five testing locations. (Map courtesy of Google satellite Maps). Samples’ locations are (A, B, C, D and E) (Abu Baker et al., 2018) |
2.2. Colilert Test
- Colilert is a commercially available enzyme-substrate liquid-broth medium (IDEXX Laboratories, Inc., Westbrook, Maine) that allows the simultaneous detection of total coliforms and Escherichia coli (E. coli). It is available in the most-probable number (MPN) or the presence/absence (PA) format. The MPN method is facilitated by use of a specially designed disposable incubation tray called the Quanti-Tray®. The PA method can be done using any sterile bottle of the appropriate size. Both methods can be done in the field or laboratory. Theory: Two enzyme substrates are included in Colilert—a chromogen that reacts with the enzyme found in total coliforms (galactosidase), and a fluorogen that reacts with an enzyme found in E. coli (glucuronidase). After 18 or 24 hours incubation at 35°C (depending on which test kit is used), a total- coliform-positive reaction turns the medium yellow; an E. coli-positive reaction causes the medium to fluoresce under a long-wave ultraviolet light (366 nm). The Colilert method is approved for use with drinking water.
2.3. Media Sources
- The Colilert substrate: Colilert, Cat WP020 (20 tests) or Cat WP200 (200 tests); incubation trays (Cat WQT100 (Quanti-Tray) or Cat WQT-2K (Quanti-Tray/2000); the Quanti-Tray sealer (Cat WQTS2X-115) is purchased from the manufacturer (IDEXX, 800/321-0207).
2.4. Analyzing Samples by Most Probable Number Method
- The sealer was warmed for 10 minutes. Undiluted sample was used. The system will enumerate between 1 and 2,400 MPN/100 mL for the undiluted sample. 100 mL of sample was combined with one packet of Colilert reagent. The reagent/sample mixture was poured into the incubation tray. The tray was run through the Quanti-Tray sealer then incubated at 35±0.5C. Colilert-24 results are definitive at 24–28 hours. In addition, positives observed before 24 hours and negatives observed after 28 hours are also valid. Count positive wells were counted based on; 1.Fluoresce under a long-wave ultraviolet light as E. coli; 2. Appear yellow under ambient light as total coliforms; 3. Dim yellow color and dim or off color fluorescence are not counted as positive results. The MPN table was used to obtain results and get MPN/100 mL.
2.5. Quality Control Cultures
- Positive and negative control cultures were performed once every 20th sample. Control cultures were ordered from the Idexx.
2.5.1. Supplies Required
- IDEXX controls: UN3373-WQC Coliform/E. coli for Colilert (IDEXX cat #: 98-29000-00); three Colilert reagent packets; Three Quanti-Tray/2000; three sterile bottles or vessels; 300 mL of sterile deionized water. One set (3 vials) should be processed per analyst. separate small bottles for were used for processing each of the three controls in the set and follow the manufacturer’s instructions. Control cultures are used to ensure that field personnel are able to correctly distinguish between positive and negative wells and process the samples correctly. For the Colilert method, three cultures are analysed: First, E. coli—positive for total coliforms and E. coli. Wells will be yellow under normal light and fluoresce under long wave UV light. Secondly, Klebsiella pneumonia—Positive control for total coliforms, but negative for E. coli. Wells are yellow under normal lighting, but do not fluoresce. And finally, Pseudomonas aeruginosa—Negative for total coliforms and E. coli but does grow and produces a faint green fluorescence. Wells are counted as negative for yellow color and fluorescence.
3. Results and Discussion
- The current study is the first research project that was conducted in this area. According to the Environmental Protection Agency (EPA), The levels of E.coli in fresh water should be less than 33 colony forming units (cfu)/100mL for a 30 day mean and 61 – 151 cfu/100 mL as a single sample reading. Our results (Table 1) are within the EPA legal limit. However, has been noticed that the two locations (Big Run Boat Camp (B) and Dillion Park Beach (C)) which have total coliform and E.coli they show high level of ammonium and nitrates. Elevated E.coli counts are because of failing home sewage treatment systems and manure application on farm fields. Although, E.coli level was legal, its presence is concerning. E. coli is a pathogenic bacterium which may cause ear, nose and throat infections as well as stomach upset and diarrhea.
4. Conclusions
- Based on the current results, it is determined that E.coli level are mostly with in the acceptable and legal limits. However, its presence is concerning. These bacteria can cause water turbidity and deplete oxygen inside water which kill water life and damage the ecosystem. Presence of such pathogens in water, warrants more effective treatment of water. Farmers shall use more environment friendly fertilizer on their farming land and utilize better filtration system to get rid of their agricultural waste. The current results show that Colibert test is sensitive, accurate and effective in measuring E.coli in water samples. The current methodology can be used in future testing of water in this area and other areas of Ohio.
5. Future Directions
- In the future, we plan to collect and test another round of samples at the same sites during the fall. We plan to do thin layer chromatography in order to determine the species of algae growing in Dillon Lake, as well as whether or not it's a threat to humans and/or wildlife.
ACKNOWLEDGEMENTS
- We would like to thank Ohio University Zanesville their support, guidance and funding throughout this project.
References
| [1] | Abu Baker, S., Russell, C. and Doudna, J. (2018). Investigating the Levels of Ammonia and Nitrates in Dillon Lake and Their Effects on the Environment. AJEE 8(1): 1-3. |
| [2] | Adams, J. C., Lytle, M.S., Dickman, D.G., Foster, D.H. Connell, J.P. and Bressler W.R. (1989). Comparison of methods for enumeration of selected coliforms exposed to ozone. Appl. Environ. Microbiol. 55: 33–35. |
| [3] | Clark, R.M., Geldreich, E.E., Fox, K.R., Rice, E.W., Johnson, C.H., Goodrich, J.A., Barnick, J.A. and Abdesaken, F. (1996). Tracking a Salmonella serovar typhimurium outbreak in Gideon, Missouri: role of contaminant propagation modelling. J. Water SRT-Aqua. 45: 171–183. |
| [4] | Colilert test, IDEXX, USA. 2019. https://www.idexx.com/en/water/water-products-services/colilert/. |
| [5] | EPA. Drinking water regulations. EPA, Environmental Protection Agency, 10 Sept. 2019, https://www.epa.gov/dwreginfo/drinking-water-regulations. |
| [6] | Geldreich, E.E., Fox, K. R., Goodrich, J.A., Rice, E.W., Clark, R.M. and Swerdlow, D.L. (1992). Searching for a water supply connection in the Cabool, Missouri disease outbreak of Escherichia coli O157:H7. Water Res. 26: 1127–1137. |
| [7] | Google Maps, https://www.google.com/maps. |
| [8] | LeChevallier, M.W. (1990). Coliform bacteria in drinking water: a review. J. AWWA 82: 74–86. |
| [9] | McFeters, G.A., Kippin, J.S. and LeChevallier, M.W. (1986). Injured coliforms in drinking water. Appl. Environ. Microbiol. 51: 1-5. |
| [10] | Rompre, A., Servais, P., Baudart, J., de Roubin, M.and Laurent, P. (2002). Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J. Microbiol. Methods. 49: 31-54. |
| [11] | Venkateswaran, K., Murakoshi, A. and Satake, M. (1996). Comparison of Commercially Available Kits with Standard Methods for the Detection of Coliforms and Escherichia coli in Foods. Appl. Enriron. Microbiol. 62(7): 2236-2243. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML