-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2019; 9(1): 6-11
doi:10.5923/j.microbiology.20190901.02

Demonstration of Pectinase Enzyme Activity from Soil Isolated Bacillus spp. in Karu Nasarawa State of Nigeria Using Orange Peels Substrate
Ajobiewe H. F.1, Ajobiewe J. O.1, 2, Mbagwu T. T.1, Ale T.2, Yakubu G. T.1
1Biological Sciences Department Bingham University Karu, Nasarawa State of Nigeria
2Microbiology Department National Hospital Abuja, FCT, Nigeria
Correspondence to: Ajobiewe J. O., Biological Sciences Department Bingham University Karu, Nasarawa State of Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Pectinases are industrially important enzymes. Bacillus spp. isolated from soil sample was used for pectinase production utilizing orange peels as its substrate under submerged fermentation. Enzyme activity Increased with increase in temperature with peak at 30°C which decreased with further increase in temperature. At pH 7, enzyme activity was optimum which decreased with increase in alkalinity, temperature and incubation time. Maximum activity of the pectinase enzyme was obtained with glucose at pH7 and temperature of 30°C. Supplementary carbon sources viz;- glucose, sucrose , lactose and orange peel were crucial for pectinase activity demonstration at 30°C. Glucose enhanced the highest pectinase activity of 54.92 U/ml. Bacillus spp. demonstrated high pectinase enzyme activity, which is an indication that this organism is of great importance for industrial pectinase enzyme activity demonstration. It is expected that with further strain improvement and substrate modification, greater activity can be achieved.
Keywords: Pectinase, Enzyme, Bacillus, Activity, Citrus, Temperature
Cite this paper: Ajobiewe H. F., Ajobiewe J. O., Mbagwu T. T., Ale T., Yakubu G. T., Demonstration of Pectinase Enzyme Activity from Soil Isolated Bacillus spp. in Karu Nasarawa State of Nigeria Using Orange Peels Substrate, Journal of Microbiology Research, Vol. 9 No. 1, 2019, pp. 6-11. doi: 10.5923/j.microbiology.20190901.02.
Article Outline
1. Background of Study
- Citrus is one of the world’s most important economic fruit crops grown all around the world. It belongs to the group of fruits that includes oranges, lemon, limes, grape fruits and tangerines (Dhillon et al., 2004). Citrus fruits are also made use of in production of squashes, citrus fruit powders, marmalade and other flavoring agents. Dried citrus peel is rich in carbohydrates, proteins and pectin; pectin acts as the inducer for production of pectinolytic enzymes by microbial systems. Processing and utilization of orange fruits into various products eventually leads to generation of waste in the form of peels, pulp and seeds. (Rajwant et al. 2004). The waste has about 66 million tons annual production and a huge amount of it is discarded to nature causing serious environmental problems (Pourbafrani et al., 2007; Tripodo et al., 2004). Orange waste is conventionally bio-transformed anaerobically into humus, although many valuable by-products can be produced from the rich waste. In other words, wealth can be derived from this waste by value addition and products such as pectin, peel oil, dietary fibers and predominantly pectinases can be easily harnessed (Bali, 2003).
2. Introduction
- Enzymes are highly efficient biological catalysts which perform all synthetic and derivative reactions in living organisms. Enzymes are the Bio-active compounds that regulate many chemical changes in living tissues (Prathyusha and Suneetha, 2011). The enzymes are preferred to chemicals in commercial endeavor mainly because of their high catalytic power, specific mode of action, stereo-specificity, eco-friendly nature and reduced energy requirement. Pectin is a heterogeneous structural polysaccharide present in primary cell wall and middle lamella of fruits and vegetables that shows wide range of industrial applications while pectinases on the other hand are a group of different enzymes that contribute to the breakdown of pectin (Voragen et al., 2003; Prathyusha and Suneetha, 2011).
3. Materials and Methods
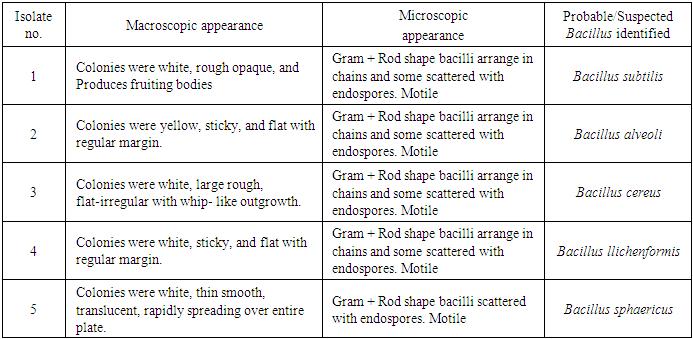
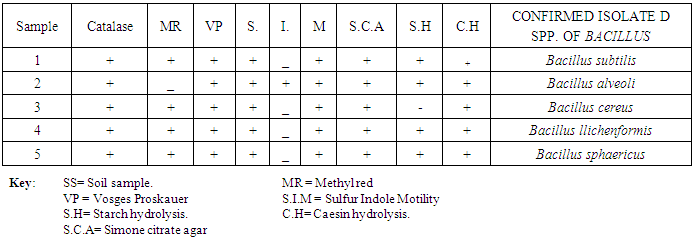
- Assorted Orange peels were randomly collected from various locations in Karu local government of Nasarawa state. They were dried in hot air oven at 40°C, ground using a mill and then stored for further use. Soil samples were similarly collected from fruit markets waste dump of Karu by putting sterile spatula through a depth of 0 – 20 cm of the soil and then transferred in to sterile zip bag. The protocol for sampling soil was carried out as described by (Ferraris, 2005). The soil samples were transported immediately to the Laboratory for analysis. Macroscopic and Microscopic identification of bacteria isolates were based on cultural and morphological characteristics consistent with the usual laboratory techniques of systematic bacteriology including those of endospore formation and staining (Hussey and Zayaitz, 2012). The Gram staining procedure as described by (Cheesbrough, 2006); Screening for Endospore as described by (Jackie and Richland, 2011) and Biochemical Test; the isolates from soil were identified by conventional biochemical tests in accordance with Bergey’s Manual of Systematic Bacteriology. Screening for pectinase producing bacteria: Isolates were screened for pectinase production, using pectinase screening plate (PSP composed of Yeast extract = 1g, Ammonium sulfate = 2g, Na2HPO4 = 6g, KH2PO4 = 3g, Citric pectin = 5g, Agar = 20g). After 24hrs incubation, the clear zones around the wells were noted when flooded with 1% cetyltrimethyl ammonium bromide to confirm the organism as a pectinase producer (Mukeshkumar et al., 2012). Quantitative Assay: the quantitative assay of pectinase was done by using pectin as substrate. The reaction mixture containing 0.5 ml of the crude enzyme and 0.5 ml of pectin in 0.1M acetate buffer with pH 6.0 was incubated at 40°C for 10 mins. 1 ml of DNS reagent was added and was boiled for 5 mins at 90°C. One milliliter of Rochelle’s salt was added to stop the reaction. The absorbance was read at 595 nm. Standard glucose solution was used to generate standard graph. One unit of Pectinase activity was defined as the amount of enzyme which liberated 1μm glucose per min. Five bacterial isolates were obtained from soil containing decaying fruits and vegetables. The criterion for the selection process was based on isolation of species with similar morphological features for the test standard culture containing citric pectin as sole source of carbon. The isolates subjected to preliminary /systematic bacteriologic examinations were numbered 1, 2, 3, 4 and 5 until finally confirmed biochemically as particular spp. of bacillus as shown in the following table of results.
4. Results
- RESULT OF ISOLATION OF BACILLUS SPECIES.Macroscopic and Microscopic Examination of Bacterial Isolates
|
|
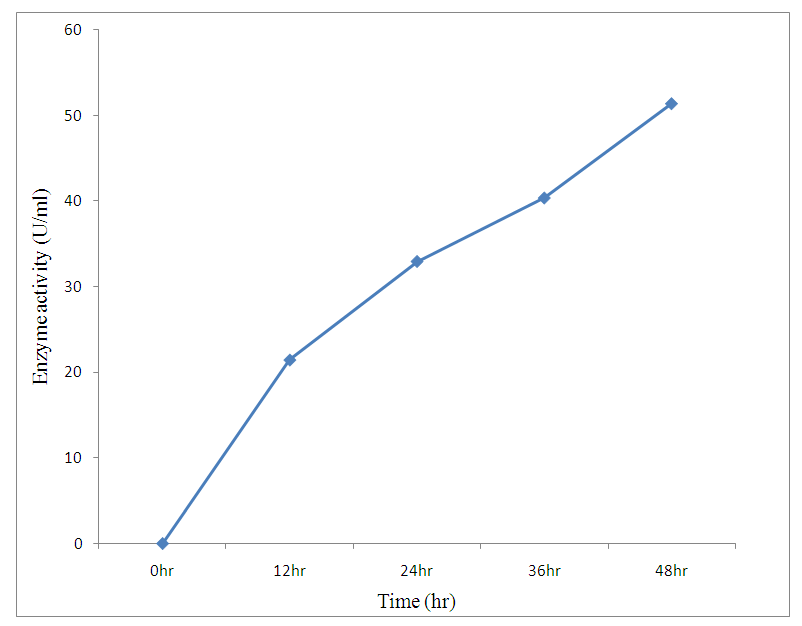 | Figure 1. Effect of incubation time |
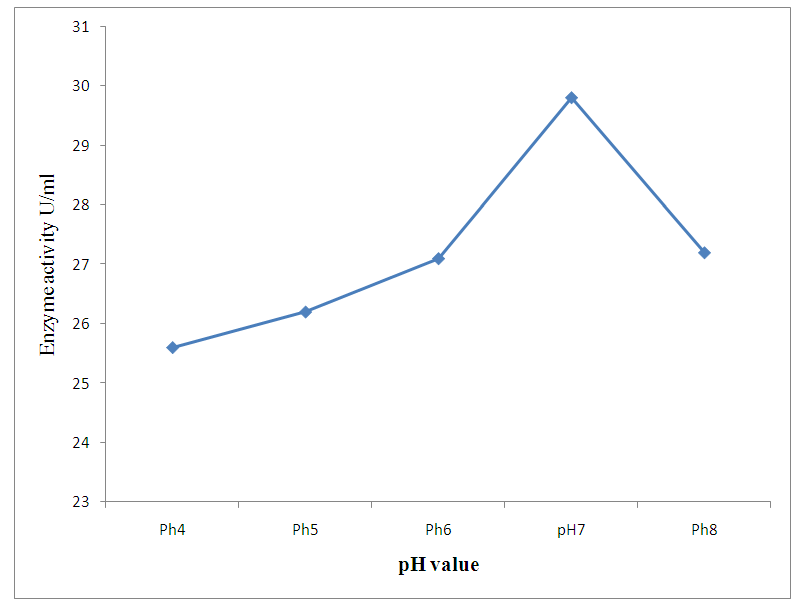 | Figure 2. Effect of pH change |
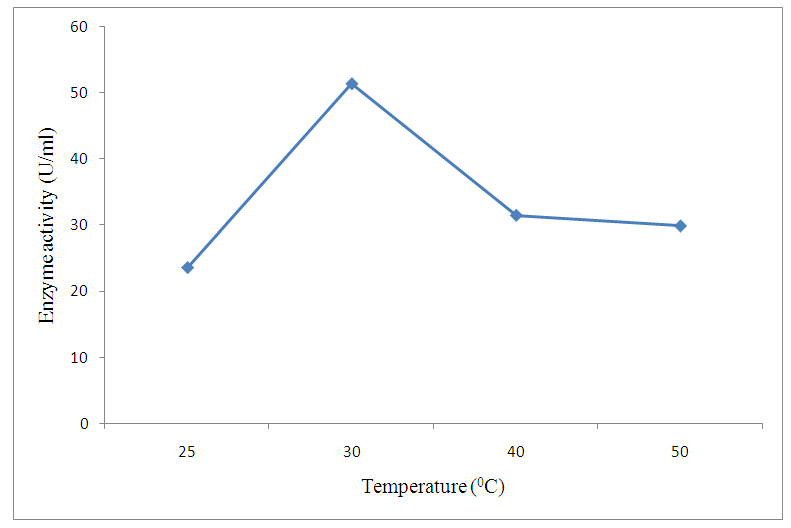 | Figure 3. Effect of temperature change |
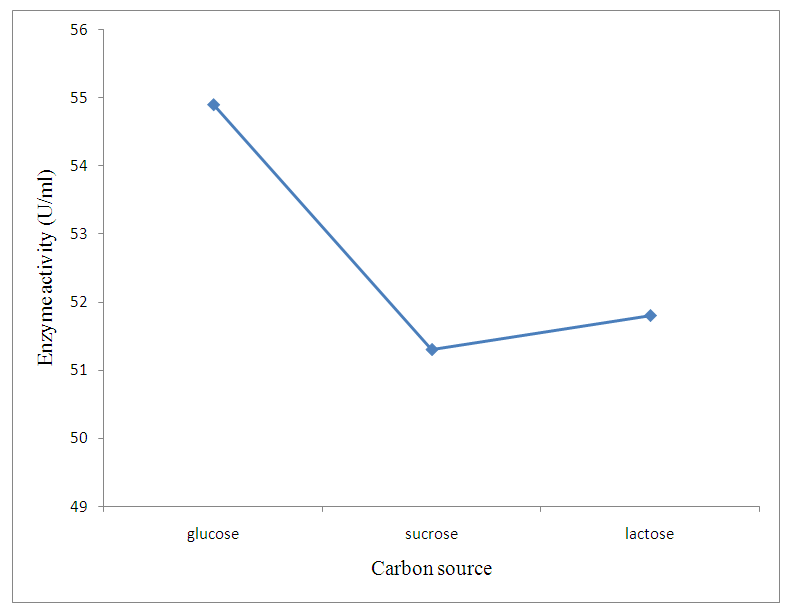 | Figure 4. Effect of different supplementary carbon source |
5. Discussion
- The preliminary study on morphology of the bacterium suggested that the bacterium is a Bacillus species. The bacterium was rod shaped and had a positive gram stain reaction. The isolates were identified by conventional biochemical tests in accordance with Bergey’s Manual of Systematic Bacteriology (Taiwo and Oso, 2004). The isolates were considered to be the members of the group Bacillus based on the staining procedures and the biochemical results (Kasing et al., 2000).Enzymes are well known biocatalyst that perform a multitude of chemical reaction and they mainly function in a narrow range of pH, temperature and ionic strength. In this present study, demonstration of pectinase activity using orange peel as substrate is important in developing enzyme systems which could be directly used for converting biomass into enzymes. Arpita et al., 2011, also stated that, when the temperature is altered below or above the optimum, there is a decrease in the activity of the enzyme and it may become denatured. This perfectly agrees with our current findings in this work.The rate of enzyme activity depends on various factors such as incubation time, temperature, pH, and carbon source (Neeta et al., 2011). Incubation time has a wide range of effect on enzyme activity; In similar fashion, the maximum enzyme activity in this work was achieved at 48hrs.of incubation. At this time the bacteria has adapted to the new environment and has enhanced enzyme activity that will result in the breakdown of the substrate. With further increase in time the enzyme activity might tend to decrease as a result of over utilization of substrate making it insufficient.The effect of pH on the pectinase activity showed that the maximum enzyme activity was at 48 hours 29.81U/ml (fig 2). Further increase in pH to 8 resulted in considerable decrease in enzyme activity. This decrease in the enzyme activity can attributed to decrease in the growth of organism at these pH values. Altering the pH above or below its optimum pH will also reduce the enzyme's activity, and at extremes the enzyme may be permanently denatured. (Baladhandayutham and Thangavelu, 2010).The effect of temperature on the pectinase activity of Bacillus. spp. was determined at various temperatures ranging from 25°C to 50°C at pH 7. An increase in temperature was accompanied by an increase in pectinase activity up to the optimal temperature of 30°C (51.3U/ml) after which the enzyme activity decreased steadily as shown in fig 3. These results indicate that the enzyme is a thermostable enzyme the decrease in enzyme activity at higher temperature may be due to enzyme denaturation (Urmila et al., 2005). Highest activity were observed with lactose and glucose. The activity of pectinase was greatly hidden when the bacterium was grown on sucrose, but pectinase activity was found to be enhanced when the bacterium was grown on lactose and glucose as stated earlier. The same results were observed by (Ranveer Singh Jayani et al., 2010).
6. Conclusions
- Bacillus spp. were isolated from the soil. Five strains, 1, 2, 3, 4 and 5 were isolated and confirmed through biochemical characterization as Bacillus subtilis, Bacillus alveoli, Bacillus cereus, Bacillus llichenformis and Bacillus sphaericus respectively. This enzyme, pectinase, produced by the individual Bacillus spp. shows maximum activity using pectin in orange peels as substrate. Results obtained from this work indicated that orange peels could be utilized to effectively demonstrate the activity of pectinases, under submerged fermentation system. Pectinases produced from this bacteria specie has an optimum pH and temperature of 7 and 30°C respectively. This can be efficiently applied industrially for various purposes such as fruit processing, vegetable oil extraction, coffee and tea fermentation, paper making and cotton fabric processing etc. Since orange peels utilized in this process are readily accessible as waste with little or no cost and also containing an appreciable amount of pectin, they can be regarded as a low-cost substrate for efficient commercial and economic reasons. Environmental pollution load resulting from these wastes (i.e. Orange peel) is similarly minimized.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML