-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2018; 8(3): 74-89
doi:10.5923/j.microbiology.20180803.04

Hazards and Prevalence of Hepatitis A virus (HAV) and Human norovirus (NoV) in Leafy Green Vegetables from Egyptian Farms
Ahmed R. Sofy 1, Khalid A. El-Dougdoug 2, Adel A. Mousa 1, Ghada S.E.A. Salem 3, Ahmed A. Hmed 1, Amr R. Ghalleb 1, 3
1Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
2Virology Lab., Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
3Food Hygiene Department, Animal Health Research Institute, Egypt
Correspondence to: Ahmed R. Sofy , Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Virus adsorption and the subsequent survival of viruses on fresh produce is of concern because of the increasing amount of fresh produce consumed in Egypt. Because fresh produce is not cooked to the same extent as meat products, vegetables provide an ideal vector for virus transmission. So, the objective of the present study was to determine the prevalence of Hepatitis A virus (HAV) and Human norovirus (NoV) which are the most commonly documented enteric viruses as contaminating vegetables. Therefore, may pose a risk to human health and causes many foodborne diseases. For this purpose, a total of 135 fresh produce samples were collected from Giza, Kafr El-Sheikh, and Qalyubia governorates. Since 45 samples from fields irrigated with water from Elmaryotia canal, 45 samples from fields irrigated with water from Kitchener drain, and 45 samples from El-Gabal El-Asfar farm, respectively. Three fresh produce items (lettuce, spinach and green onions) were chosen for this study, where 15 samples from each item. The obtained results revealed that fresh produce samples which collected from the three governorates showed high contamination percentages by HAV and NoV. Samples collected from the fields irrigated with water from Kitchener drain (Kafr El-Sheikh governorate) showed the highest contamination percentages by 73.3%, 60% and 46.6% for lettuce, spinach, and green onion, respectively for HAV. Towards NoV the percentages were 26.6%, 26.6% and 6.6%, respectively. As well as, the contamination percentages of the previous three items collected from El-Gabal El-Asfar farm (Qalyubia governorate) for HAV were 60%, 40%, and 26.6%, respectively. Whereas the percentages for NoV were 20% for both lettuce and spinach. NoV not detected in any samples examined of green onion collected from this region. On the hand, samples of Elmaryotia canal (Giza governorate) showed the lowest contamination percentages, where both viruses not detected in green onion examined samples. Further, HAV was only detected in lettuce plant samples by 20%, while spinach samples showed contamination with HAV and NoV by 33.3% and 13.3%, respectively. Lettuce samples from the three regions showed the highest contamination percentages with HAV followed by NoV. But in contrast, the green onion examined samples from the previous three regions showed the lowest contamination percentages.
Keywords: HAV, NoV, Fresh produce, Lettuce, Spinach, Green onion, Egypt
Cite this paper: Ahmed R. Sofy , Khalid A. El-Dougdoug , Adel A. Mousa , Ghada S.E.A. Salem , Ahmed A. Hmed , Amr R. Ghalleb , Hazards and Prevalence of Hepatitis A virus (HAV) and Human norovirus (NoV) in Leafy Green Vegetables from Egyptian Farms, Journal of Microbiology Research, Vol. 8 No. 3, 2018, pp. 74-89. doi: 10.5923/j.microbiology.20180803.04.
Article Outline
1. Introduction
- Viruses have been implicated in some foodborne illnesses and have become a significant threat to public health [1, 2]. Among foodborne viruses, Norovirus and Hepatitis A virus are the most important foodborne viruses, due to a large number of outbreaks caused by Norovirus, and the severity of hepatitis a contributing to higher hospitalization and death rates [3].Hepatitis A virus (HAV) is one of the most widespread foodborne pathogens and the cause of viral hepatitis. Fresh foods can be considered as a vector of transmission for HAV when contaminated by spoiled irrigation water or when prepared by infected food handlers. HAV is among those enteric viruses that can be transmitted by contaminated food. Over 95% of HAV infections are transmitted by the fecal-oral route [4], and person-to-person contact is considered to be the primary mode of transmission [1]. Recent food-borne outbreaks of hepatitis A have been associated with different types of canned soft fruit like strawberries [5], vegetables like green onions and lettuces [6] and frozen berries [7]. HAV is considered one of the most common illnesses through oral-fecal infection special in children and old’s in the poor developing country [8].Noroviruses (formerly named Norwalk-like viruses (NVLs) or small round structured viruses (SRSVs)) are commonly associated with most foodborne viral outbreaks. The Center for Disease Control and Prevention (CDC) estimates that more than 21 million cases of acute gastroenteritis occur each year due to Noroviruses infection, and 58% of all foodborne disease outbreaks can be attributed to Noroviruses [9]. This makes Noroviruses the leading of most common cause of gastroenteritis.Noroviruses (NoVs), members of the Caliciviridae family, are small, positive-polarity RNA viruses and were recently recognized as the second most common cause of severe childhood gastroenteritis [10]. Worldwide, NoVs are responsible for almost 50% of AGE outbreaks and more than 90% of the non-bacterial gastroenteritis, considered the major pathogens in outbreaks [11]. Human noroviruses and Hepatitis A virus are now recognized as significant etiologies causing produce-associated outbreaks [12]. However, fresh produce, usually consumed raw or uncooked, can serve as vehicles for the transmission of these viruses and may pose a risk for human health and considered the sources of many foodborne diseases. Therefore, the objective of the present study was to determine the prevalence of HAV and NoV from the most cultivated vegetable items (lettuce, spinach and green onion) collected from different governorates in Egypt which may pose a risk for human health to be taken.
2. Materials and Methods
2.1. Collection of Samples
- A total of 135 fresh produce samples were collected from three governorates [45 samples from fields irrigated with water from Elmaryotia canal (Giza governorate), 45 samples from fields irrigated with water from Kitchener drain (Kafr El-Sheikh governorate), and 45 samples from El-Gabal El-Asfar farm (Qalyubia governorate)]. Because of their previous epidemiological association with foodborne HAV and NoV outbreaks, three produce items (lettuce, spinach and green onions) were chosen for this study, 15 samples from each item. The samples of each item were kept in a plastic bag and stored at (-20ºC) until use for examination and further studies.
2.2. Isolation of Virus
2.2.1. Pre-Analytical Sample Processing
- Fresh plant tissues (2 kg) were sterilized by soaking in 70% ethanol for 5 min then socked in 2% sodium hypochlorite for 1 min, washed in sterilized distilled water for 2 min and dried. To investigate the presence of HAV and NoV in the samples, fresh plant tissues of each sample was cut and mixed in sterile distilled water and macerated using an electric blender. The extraction was filtrated with two layers cheesecloth. The crude sap was clarified by filter paper (Whatman No 4) and stored at -20oC until used for concentration.
2.2.2. Concentration of Virus
- Primary concentration: Viruses were eluted and concentrated from foods surface and crude sap samples via 150 ml of 10% (w/v) beef extract was added to 100 g raw fresh foods and to 100 ml crude sap samples, the mixtures were stirred for 30 min at room temperature and centrifuged at 12000 rpm for 15 min at room temperature [13]. The pellet was discarded, and the supernatant was then concentrated by organic flocculation method.Secondary concentration: All samples were secondary re-concentrated using an organic flocculation method according to Anonymous [5], Katzenelson et al. [14], and Mäde et al. [15], then the elutes were acidified to pH 3.5 using HCl (5N), (Merck-Schuchardt) and centrifuged at 3000 rpm for 15 min. The supernatant was discarded, and the pellet was dissolved in 1ml of Na2HPO4 (El Nasr Pharmaceutical Chemical Co.). All samples were kept at -70oC for further analysis.
2.3. HAV Detection
2.3.1. Reverse Transcription-PCR (RT-PCR)
- Viral RNA was extracted directly from concentrated extracts of vegetable samples using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. RT-PCR amplified the VP1-2A junction region of HAV with primers that flanked the VP1-2A junction region [16]: HAV-F2808 5′- GGTTTCTATTCAGATTGCAAATTA-3′ (nucleotides 2808–2831, position correspond to wild-type HAV, GenBank accession number M14707), and HAV-R3292 5′- AGTAAAAACTCCAGCATCCATTTC-3′ (nucleotides 3315–3292, position correspond to wild-type HAV, GenBank accession number M14707). cDNA synthesis was performed with about 100 ng of RNA using 0.1 μM of the anti-sense HAV-R primer and the M-MLV RT enzyme (Promega, Cat #), according to the manufacturer’s instructions. PCR amplification was performed according to Lee et al. [16] in a final volume of 25 μl as follows: 2.5 μl of cDNA, 2.5 μl of 2.5 mM of dNTPs, 2.5 μl of 10X buffer, 2.5 μl of 25 mM MgCl2, 1 μl of each forward and reverse primers at 10 μM, 0.2 μl Taq DNA polymerase, and water. Mixtures were incubated for 2 min at 95°C, followed by 40 cycles of 1 min at 95°C, 1 min at 55°C and 1 min at 72°C, with a final incubation of 10 min at 72°C, and then, the reaction was held at 4°C. PCR products were visualized on an ethidium bromide-stained 1.5% agarose gel.
2.4. NoV Detection
2.4.1. RT-PCR
- Viral RNA was extracted directly from concentrated extracts of vegetable samples using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Extracted samples RNA were analyzed by nested PCR using primers that flanked the RdRp gene: MR3/MR4 [17] (MR34485 5′-CCGTCAGAGTGGGTATGAA-3′ and MR44954 5′-AGTGGGTTTGAGGCCGTA-3′) for the first PCR, and Yuri22F/Yuri22R [18] (Yuri22F4505 5′-ATGAATGAGGATGGACCCAT-3′ Yuri22R4877 5′-CATCATCCCCGTAGAAAGAT-3′) for the nested PCR. RT-PCR amplification was carried out as described by Kojima et al. [19]. PCR products were visualized on an ethidium bromide-stained 1.5% agarose gel.
2.5. Statistical Analyses
- All statistical calculations were done using SPSS (statistical package for the social science version 25.00) statistical program at the 0.05 level of probability [20]. Qualitative data were done using Chi-square and Pearson correlation. The confidence interval was set to 95% and the margin of error accepted was set to 5%. The P-value was considered non-significant (NS) at the level of >0.05, and significant at the level of <0.05.
3. Results
- A total of 135 leafy green vegetable samples from three governorates (Giza, Kafr El-Sheikh, and Qalyubia) were collected to study the prevalence of enteric viruses (Hepatitis A virus and Human norovirus) in Egypt. Since 45 samples from fields irrigated with water from Elmaryotia canal (Giza), 45 samples from fields irrigated with water from Kitchener drain (Kafr El-Sheikh), and 45 samples from El-Gabal El-Asfar farm (Qalyubia). The vegetable items which chosen from each studied region were lettuce, spinach, and green onions, where 15 samples from each item.
3.1. Prevalence of Hepatitis A virus
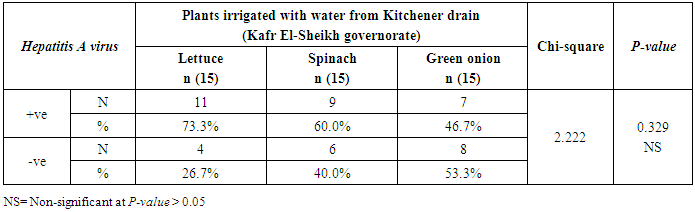
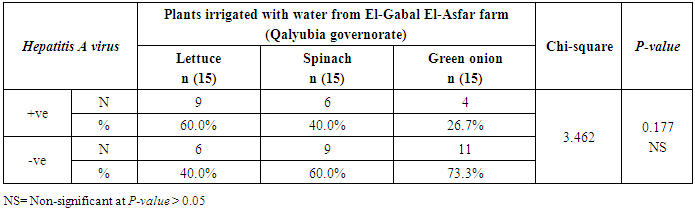
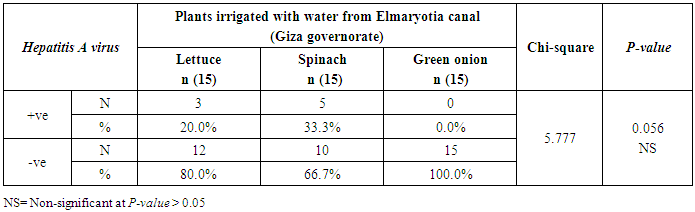
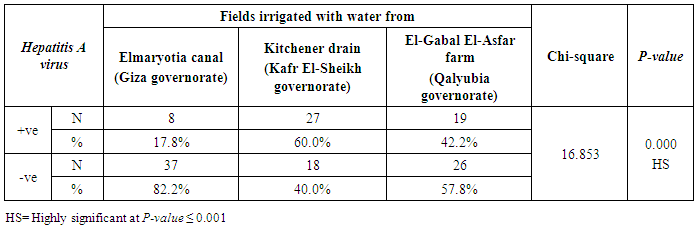
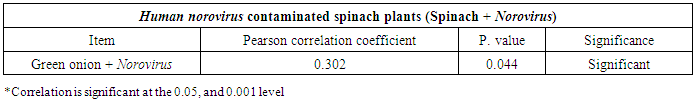
- The results showed that the highest prevalence contamination percentages of lettuce, spinach and green onion plants with Hepatitis A virus were 73.3% (11/15), 60% (9/15) and 46.7% (7/15), respectively in the fields irrigated with water from Kitchener drain (Kafr El-Sheikh governorate) (Table 1 & Fig. 1). Follows this the prevalence contamination percentages in the areas irrigated with water from El-Gabal El-Asfar farm (Qalyubia governorate), which were 60% (9/15), 40% (6/15) and 26.7% (4/15), respectively (Table 2 & Fig. 2). Whereas the lowest prevalence contamination percentages were found in the fields irrigated with water from Elmaryotia canal (Giza governorate), which were 20% (3/15), 33.3% (5/15) in case of lettuce, and spinach, respectively (Table 3 & Fig. 3). While HAV not detected in green onion examined samples collected from the fields irrigated with water from Elmaryotia canal (Table 3 & Fig. 3). So, the highest prevalence of Hepatitis A virus was found in Kafr El-Sheikh governorate by 60% (27/45), followed by 42.2% (19/45) in Qalyubia governorate, and 17.8% (8/45) in Giza governorate with high statistically significant difference between locations regarding HAV (p ≤ 0.001) (Table 4 & Fig. 4). The results revealed no statistically significant difference between HAV contaminated plants irrigated with water from Kitchener drain (Kafr El-Sheikh governorate), El-Gabal El-Asfar farm (Qalyubia governorate), or Elmaryotia canal (Giza governorate) (p > 0.05). On the other hand, the results revealed the statistically significant difference between locations regarding Hepatitis A virus present on lettuce (p ≤ 0.05), and green onion plants (p ≤ 0.05), while the non-significant difference between locations regarding HAV present on spinach plants (p > 0.05).
|
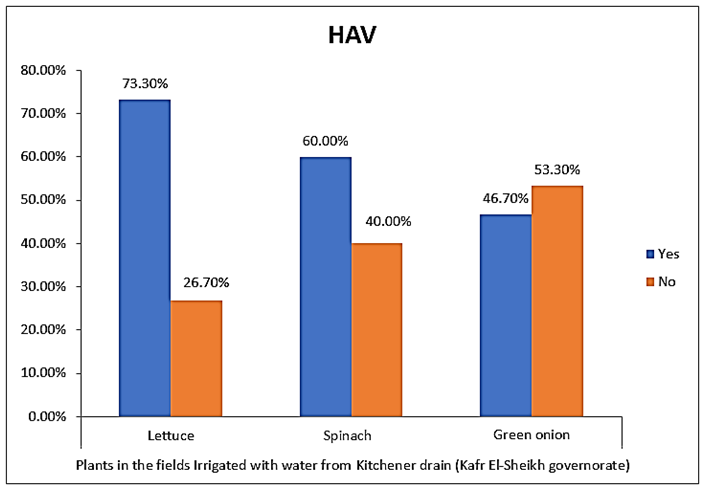 | Figure (1). Comparison between HAV contaminated plants irrigated with water from Kitchener drain (Kafr El-Sheikh governorate) |
|
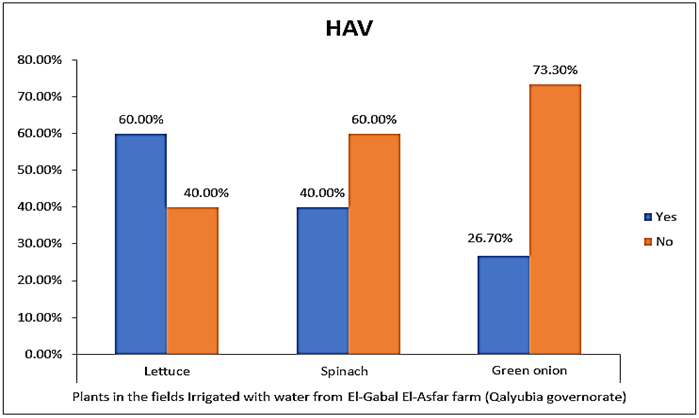 | Figure (2). Comparison between HAV contaminated plants irrigated with water from El-Gabal El-Asfar farm (Qalyubia governorate) |
|
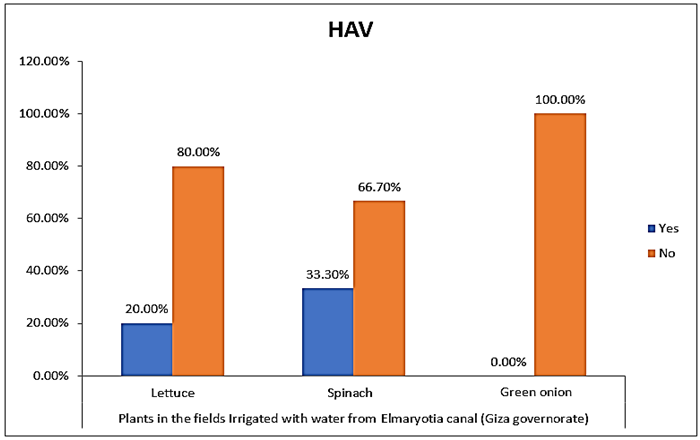 | Figure (3). Comparison between HAV contaminated plants irrigated with water from Elmaryotia canal (Giza governorate) |
|
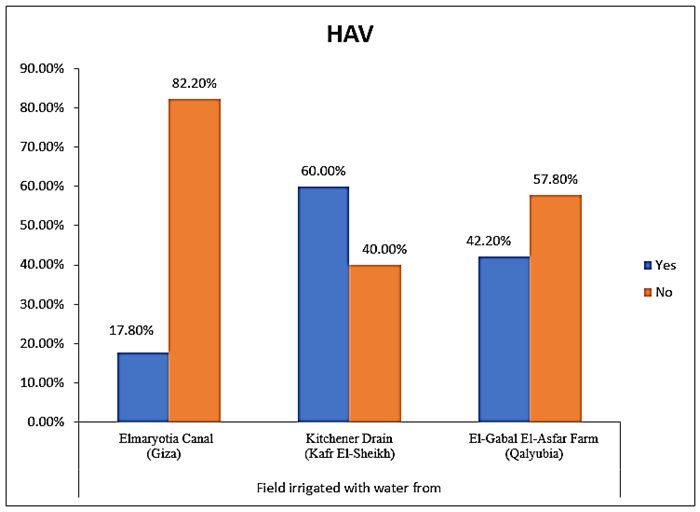 | Figure (4). Comparison between locations regarding Hepatitis A virus |
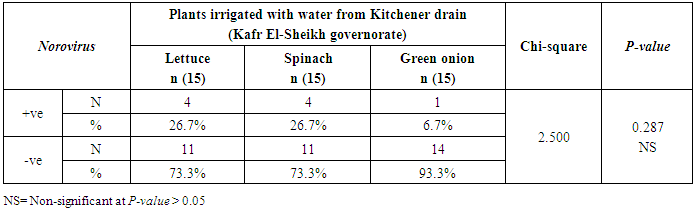
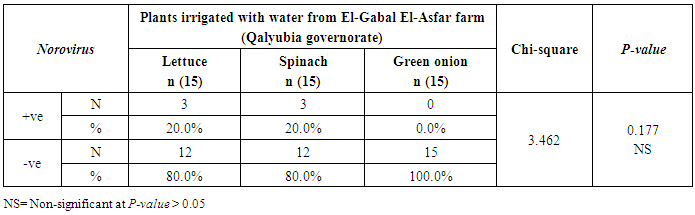
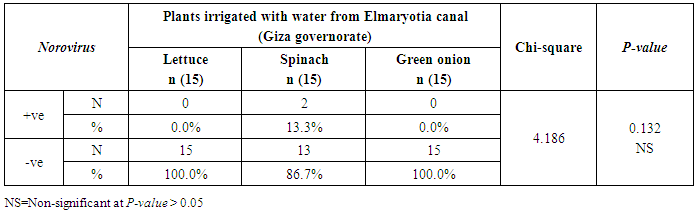
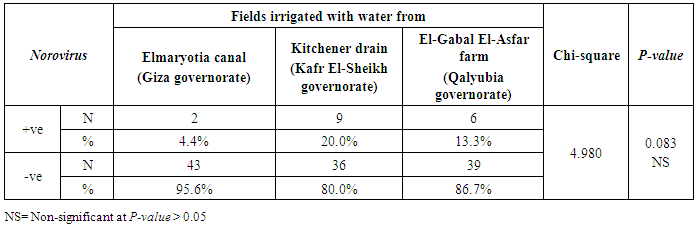
3.2. Prevalence of Human norovirus (NoV)
- The results showed that the highest prevalence contamination percentages of lettuce, spinach and green onion plants with Human norovirus were 26.7% (4/15), 26.7% (4/15) and 6.7% (1/15), respectively in the fields irrigated with water from Kitchener drain (Kafr El-Sheikh governorate) (Table 5 & Fig. 5). Follows this the prevalence contamination percentage in the fields irrigated with water from El-Gabal El-Asfar farm (Qalyubia governorate), which was 20% (3/15) in case of lettuce and spinach plants, while NoV not detected in green onion plants (Table 6 & Fig. 6). Whereas the lowest prevalence contamination percentage was found in the fields irrigated with water from Elmaryotia canal (Giza governorate), which was 13.3% (2/15) in case of spinach plants, while NoV not detected in lettuce and green onion plants (Table 7 & Fig. 7). So, the highest prevalence of Human norovirus was found in Kafr El-Sheikh governorate by 20% (9/45), followed by 13.3% (6/45) in Qalyubia governorate, and 4.4% (2/45) in Giza governorate with no statistically significant difference between locations regarding NoV (p > 0.05) (Table 8 & Fig. 8). The results revealed no statistically significant difference between NoV contaminated plants irrigated with water from Kitchener drain (Kafr El-Sheikh governorate), El-Gabal El-Asfar farm (Qalyubia governorate), or Elmaryotia canal (Giza governorate) (p > 0.05). Also, the results revealed no statistically significant difference between locations regarding Human norovirus present on lettuce (p > 0.05), spinach (p > 0.05), and green onion plants (p > 0.05).
|
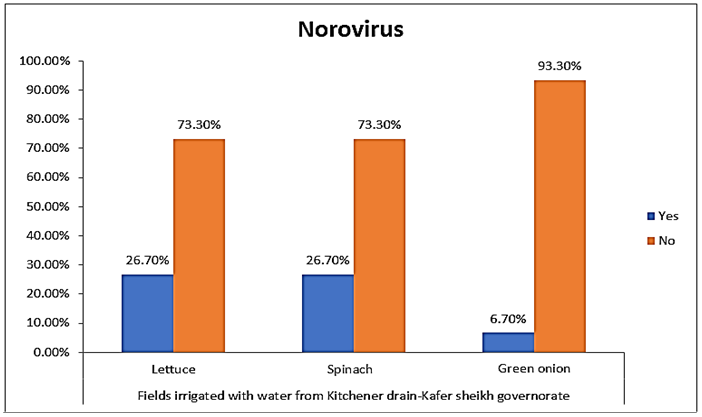 | Figure (5). Comparison between Human norovirus contaminated plants irrigated with water from Kitchener drain (Kafr El-Sheikh governorate) |
|
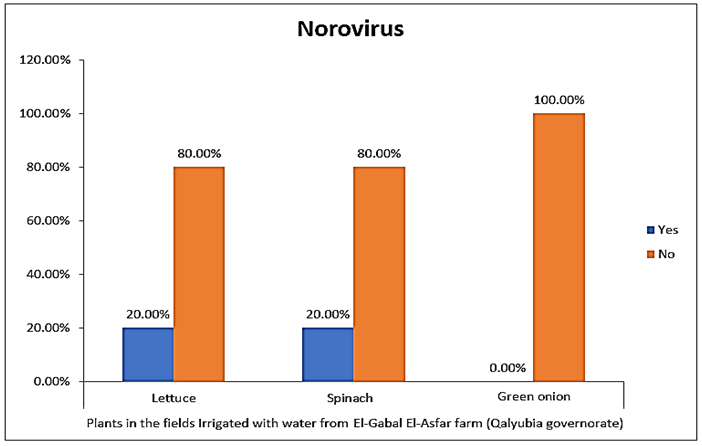 | Figure (6). Comparison between Human norovirus contaminated plants irrigated with water from El-Gabal El-Asfar farm (Qalyubia governorate) |
|
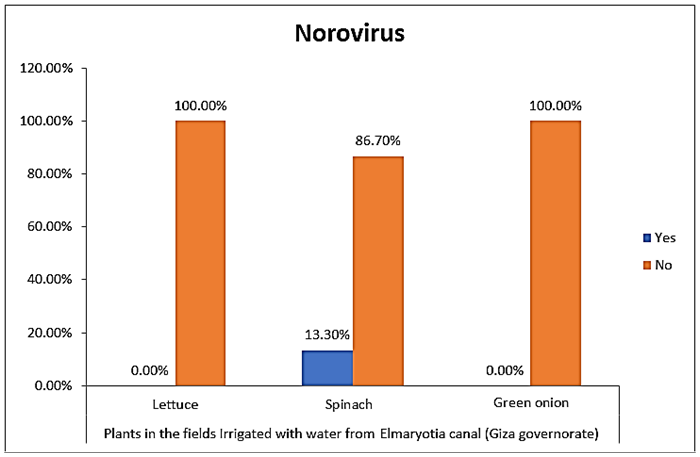 | Figure (7). Comparison between Human norovirus contaminated plants irrigated with water from Elmaryotia canal (Giza governorate) |
|
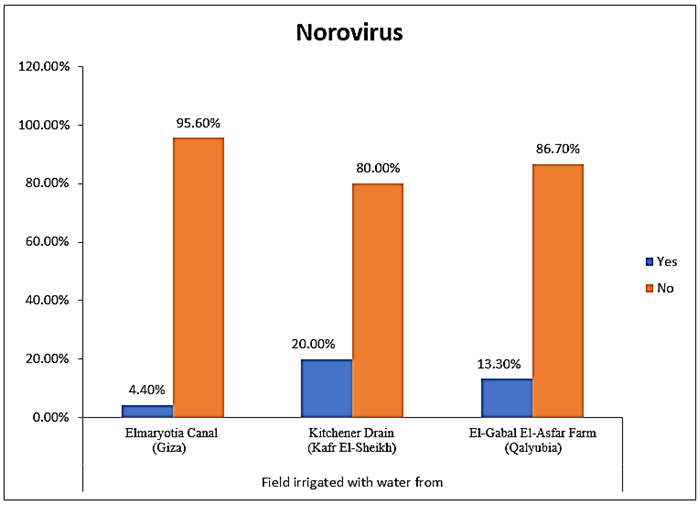 | Figure (8). Comparison between locations regarding Human norovirus |
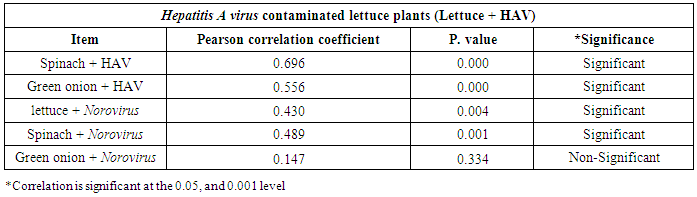
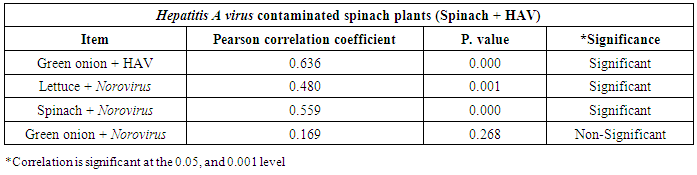
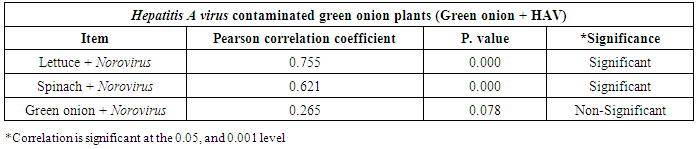
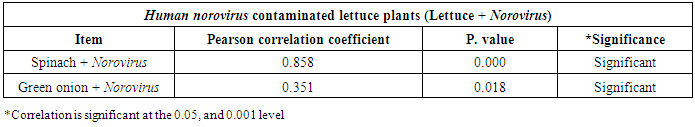
3.3. Correlation between Hepatitis A virus and Human norovirus
- As shown in tables (9, 10, 11, 12 & 13), there were statistically significant positive correlations as the following: First correlations were between Hepatitis A virus contaminated lettuce plants with HAV contaminated spinach plants (Fig. 9-A), with HAV contaminated green onion plants (Fig. 9-B), with Norovirus contaminated lettuce plants (Fig. 9-C), and with Norovirus contaminated spinach plants (Fig. 9-D). Second correlations were between Hepatitis A virus contaminated spinach plants with HAV contaminated green onion plants (Fig. 10-A), with Norovirus contaminated lettuce plants (Fig. 10-B), and with Norovirus contaminated spinach plants (Fig. 10-C). Third correlations were between Hepatitis A virus contaminated green onion plants with Norovirus contaminated lettuce plants (Fig. 11-A), and with Norovirus contaminated spinach plants (Fig. 11-B). Fourth correlations were between Human norovirus contaminated lettuce plants with Norovirus contaminated spinach plants (Fig. 12-A), and with Norovirus contaminated green onion plants (Fig. 12-B). Finally, the last correlation between Human norovirus contaminated spinach plants with Norovirus contaminated green onion plants (Fig. 13).
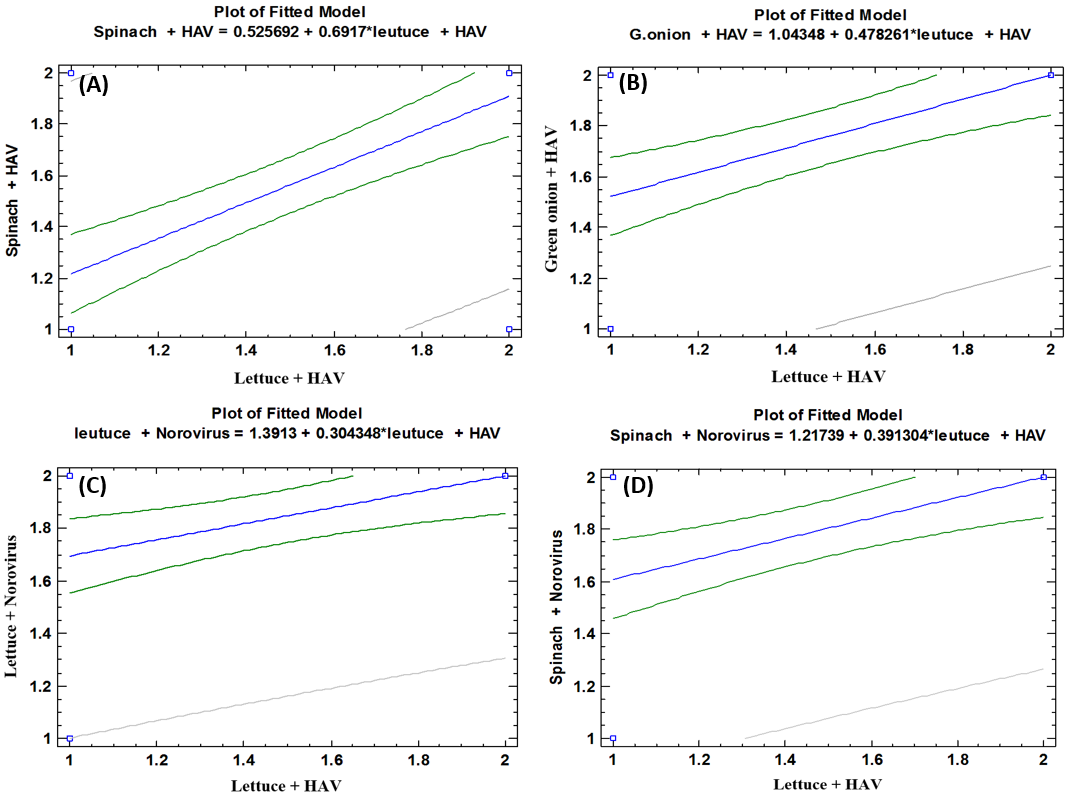 | Figure (9). Linear Pearson Correlation between Hepatitis A virus contaminated lettuce plants with HAV contaminated spinach plants (A), with HAV contaminated green onion plants (B), with Norovirus contaminated lettuce plants (C), and with Norovirus contaminated spinach plants (D) |
|
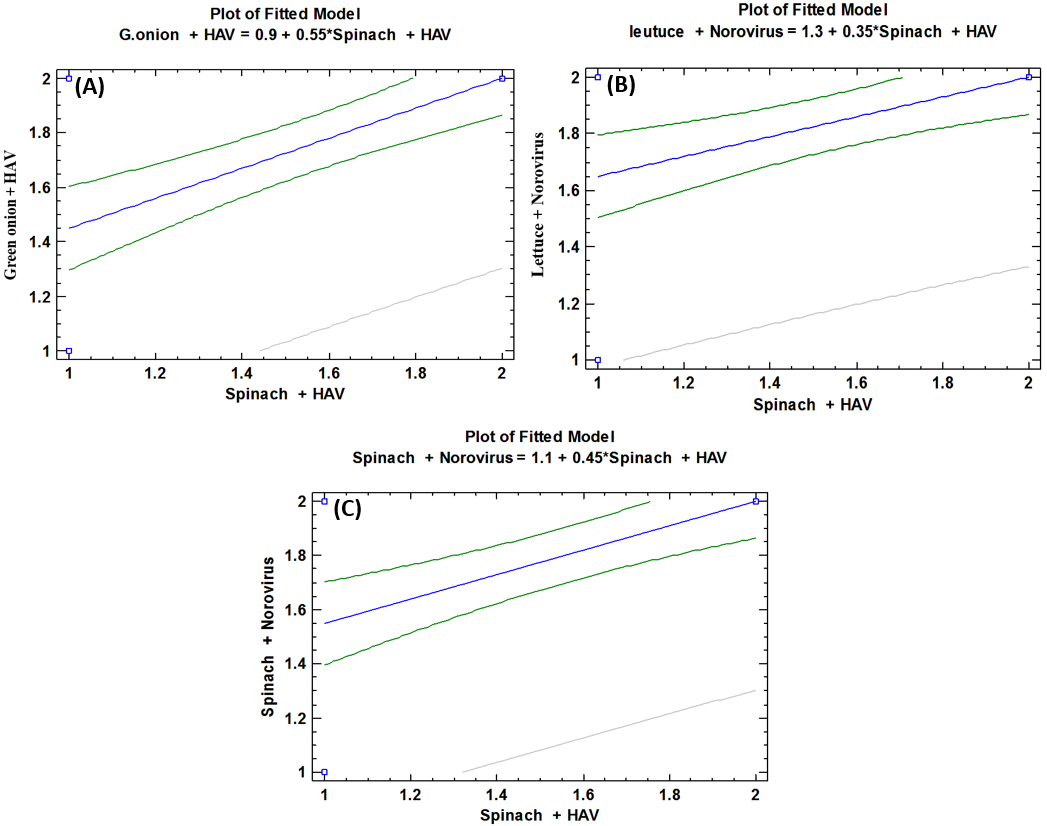 | Figure (10). Linear Pearson Correlation between Hepatitis A virus contaminated spinach plants with HAV contaminated green onion plants (A), with Norovirus contaminated lettuce plants (B), and with Norovirus contaminated spinach plants (C) |
|
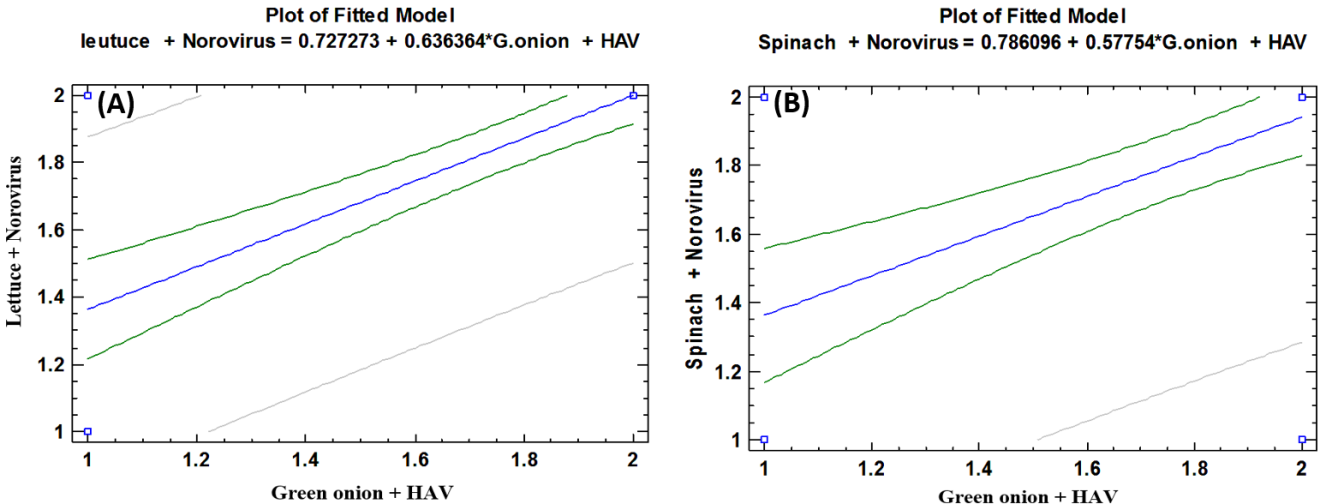 | Figure (11). Linear Pearson Correlation between Hepatitis A virus contaminated green onion plants with Norovirus contaminated lettuce plants (A), and with Norovirus contaminated spinach plants (B) |
|
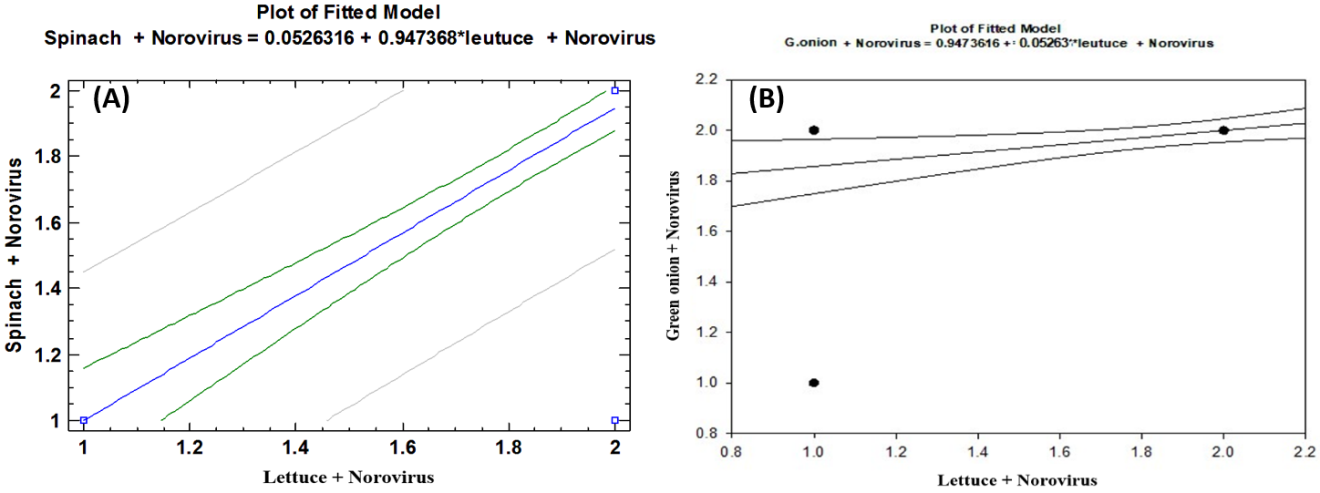 | Figure (12). Linear Pearson Correlation between Human norovirus contaminated lettuce plants with Norovirus contaminated spinach plants (A), and with Norovirus contaminated green onion plants (B) |
|
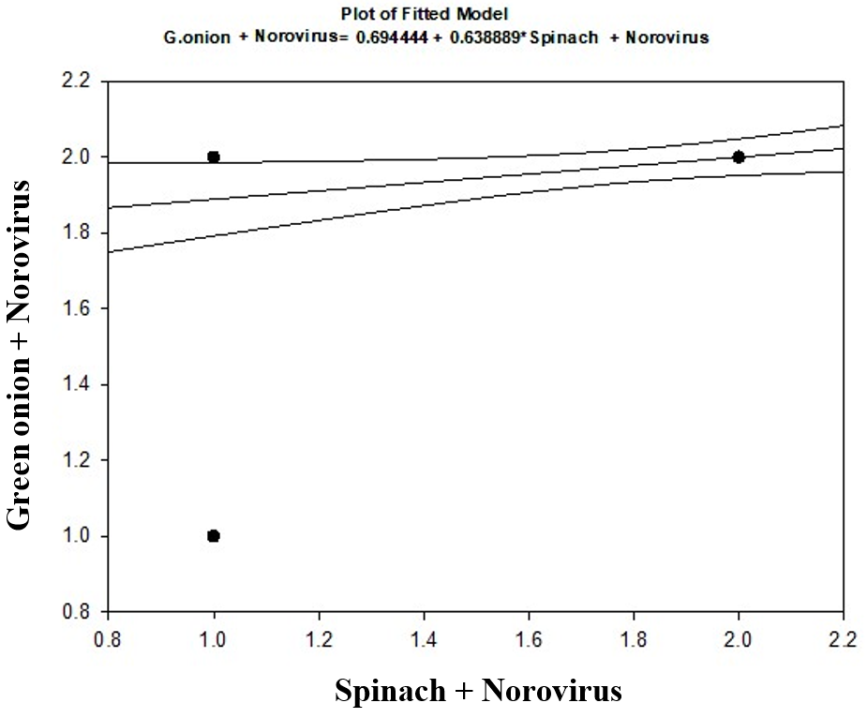 | Figure (13). Linear Pearson Correlation between Human norovirus contaminated spinach plants with Norovirus contaminated green onion plants |
4. Discussion
- Some viral foodborne disease outbreaks associated with the consumption of contaminated raw produce have occurred over the last several years, presumably due to the combined effect of increased use and better epidemiological surveillance [21]. Of the viral agents, Hepatitis A virus (HAV) and Norovirus (NoV) are those most commonly documented as contaminating fruits and vegetables. Adsorption and the subsequent survival of viruses on fresh produce are of concern because of the increasing amount of fresh produce consumed in Egypt. Because fresh food is not cooked to the same extent as meat products, lettuce, spinach and green onion and provide an ideal vector for virus transmission [22-26].The obtained results showed that HAV and NoV contaminated the examined vegetable samples. Fresh produce samples which collected from three governorates showed high contamination percentages, especially the samples collected from the fields irrigated from Kitchener drain (Kafr El-Sheikh governorate). Then the samples from El-Gabal El-Asfar farm (Qalyubia governorate). The fresh produce samples from fields irrigated with water from Elmaryotia canal (Giza governorate) also, showed contamination percentages but in little values than the other previous regions. As well as, our results showed that, the lettuce samples from the three areas showed the highest contamination percentage with HAV followed by NoV. Then the spinach samples; but in contrast, the green onion examined samples from the previous three regions showed the lowest contamination percentages.Our results are in matching with many other previous studies concerned with the detection of these viruses in fresh produce. Since Kamel et al. [22] recorded the prevalence of enteroviruses, Noroviruses, and rotaviruses using RT-PCR with 22%, 18%, and 8.3%, respectively in seventy-two sewage samples of Greater Cairo collected from two wastewater treatment plants, which were obtained from April 2006 through February 2007. El-Senousy et al. [23] reported that Norovirus still has a low prevalence in Egyptian naturally contaminated irrigation water and fresh produce, where found rotaviruses are more frequent than Noroviruses in groundwater of rural areas of Giza Governorate [24]. Also, El-Senousy et al. [25] recorded the prevalence of rotaviruses, astroviruses and Noroviruses in the raw sewage samples collected from Meet Khames wastewater treatment plant (El-Mansoura city & El-Daqahlia governorates) from March 2008 to September 2009, where the percentages of detection of RNA enteric viruses were 68.4%, 47.3%, and 5.2%, respectively using polyethylene glycol method as a secondary concentration step. On the other hand, El-Dougdoug et al. [26] have detected a Hepatitis A virus using ELISA and RT-PCR in two outlet drainage water samples from Elmaryotia canal (Giza governorate) and El-Gabal El-Asfar farm (Qalyubia governorate), which collected in each month starting from March 2015 over a period of 12 months. As well as in lettuce, green onion and strawberry samples, which were irrigated and washed with these drainage water. They found, concentrated drainage water, strawberry, and lettuce fresh food samples irrigated and washed with drainage water from Elmaryotia canal (Giza governorate) gave positive results. Whereas lettuce washed with drainage water from El-Gable Elasfar Qalyubia governorate) gave a positive result, while strawberry and green onion gave negative reaction [26].Guevremont et al. [27] presented a protocol that involved direct RNA isolation from produce tissues without any pre-concentration scheme and was able to detect less than 1 PFU of HAV per g of cilantro. Dubois et al. [28] were able to detect as little as 7 TCID50 of HAV from 100 g of fruits and vegetables with an approach involving elution of virus with a high pH buffer, followed by an overnight 10% PEG precipitation and molecular detection by RT-seminested PCR. As well as, Guevremont et al. [27] reported detection limits of 1 TCID50 HAV per 25 g of fresh green onions when using a virus elution-concentration method.Fruits and vegetables are a major vehicle for transmission of foodborne enteric viruses since they are easily contaminated at pre- and post-harvest stages and undergo little or no processing. This allows for contamination anywhere from pre-harvest to post-harvest stages [29]. Foods commonly contaminated with foodborne viruses include shellfish, fresh produce, fruits and juices, and other foods that do not undergo further thermal processing [30]. The smooth texture of the lettuce and onion leaves leads the virus may have become bound or adsorbed to the leaves surface [30]. The smooth texture of the lettuce leaf leads researchers to assume that virus particles can be easily washed from the surface for detection. This may not be the case; instead, the virus may have become bound or adsorbed to the lettuce leaf surface [31].Virus survival rates were variable depending on the produce type and typically ranged from 0.5% to 13% of the initial titers of virus inoculated on produce. The rates of virus survival can be affected by unique surfaces properties of produce items and by virus-specific properties [31]. Significantly more viruses were removed with nylon brushes from honeydew melons than from cantaloupes. This may be due to the weak attachment of the virus to the smooth surfaces of the honeydew melons or due to the physical features of the cantaloupe (roughness and absorptive nature) making it difficult to elute virus as previously discussed [32]. Different interactions have been shown to be a factor affecting virus attachment to produce [33, 34]. For example, the electrostatic and hydrophobic interactions between viruses and surfaces were observed [32, 35].One major route with a high probability of contamination is the use of contaminated water for irrigation or washing. Contamination may also occur by infected workers handling the food during harvesting, processing, or distribution. With an increasing number of people striving to eat healthier by increasing their consumption of fruits and vegetables, this has become a major public health concern [29]. The viruses inoculated to fresh produce were also found to cross-contaminate kitchen utensils, such as knives and graters [36].Use of wastewater for spray irrigation may be particularly risky as this may facilitate virus attachment to product surfaces [37]. Green onions and other select produce items may also be particularly prone to virus contamination because their surfaces are involved, allowing fecal matter and other organic materials to adhere tenaciously [21]. The survival of viruses on vegetables has been shown to be dependent upon pH, moisture content, and temperature [38]. Since the Noroviruses and HAV have been associated with some produce-associated outbreaks, it seems possible, though not yet supported by studies, that these viruses can be resistant to some of the virucidal substances naturally found in produce such as organic acids, phenolic and sulphur compounds [39]. Items such as green onions, which have recently been implicated in HAV outbreaks in the U.S., may become contaminated at any time during the production and processing continuum by contaminated soils, water, or human handling. However, as this particular product requires extensive human handling during harvesting, it has been suggested that human handling is perhaps the most likely source of virus contamination [40].Also, surface morphology and physiologic characteristics of the produce item(s) certainly complicate disinfection efficacy; leafy vegetables can be more difficult to decontaminate because of their rough or wrinkled surfaces, and small fruits like raspberries and blackberries have more porous and complex surfaces that can entrap virus particles [37]. Viral contamination also occurs when food handlers do not practice proper hand hygiene. During food handling, viruses from hands were found to transfer to stainless steel surfaces and foods (blueberries, grapes, raspberries, ham), and the transfer of viruses increased when hands were wet [41].Foods are also prone to viral contamination if exposed to virally contaminated contact surfaces, with the highest risk for foods that require minimal or no cooking before consumption (lettuce, deli meats, etc.) [42]. The attachment of the virus to vegetables is dependent on the pathogen itself, the type of fresh produce, and the surfaces investigated. For leafy greens, the surface of the leaf is easily contaminated by Norovirus, although uptake by the roots of plants can potentially occur. Fruits may be directly contaminated by Norovirus since there is no direct evidence support that pathogens in roots can be transported into fruits. There are six main pathways by which contamination may occur: 1) direct contact of produce with the ground, 2) direct contact of produce with human waste and animal contaminants, 3) dislodgment of contaminated soil through irrigation water splashing or rain onto produce, 4) aerosolization of pathogens onto produce, 5) direct application of contamination through spraying irrigation water onto the produce, and 6) contamination of fresh produce by farmers, processors, and food handlers [43].It is known that viruses and other pathogens attach to the surface of produce, but the mechanism of how they attach is still unclear. There have been some proposed mechanisms including extracellular polymeric substances, whether fimbriae are present or not, cell surface hydrophobicity, divalent cationic bridges and surface charge of the pathogens [44].Many previous studies found that enteric viruses do not employ specific cell-surface receptors to attach to the fresh produce surface. Moreover, the non-specific adsorption of enteric viruses to minimally processed fresh produce may result in either large outbreaks or sporadic outbreaks which would be difficult to trace. So, the identification of forces critical for virus adsorption is important for vegetable and fruit processing for viruses or as a preventive measure to avoid foodborne disease caused by viral agents. Additionally, other authors have found high levels of enteric viruses in discharged water from wastewater treatment plants [45-47]. The ability of human pathogenic viruses to adsorb to lettuce is of great concern given the amount of virus in waters that may not be treated before irrigation.For interpretation of the adsorption of viruses to the different surfaces including vegetables, it was postulated that two processes are involved in the formation of the adsorption equilibrium with a solid surface namely, mass transport of viruses close to the surface and secondly the immobilization of the viruses to the surfaces by physical and chemical interactions. Electrostatic interactions, van der Waals forces, and hydrophobic effects are three primary forces that are thought to be responsible for the interactions between virus particles and solid substrates [48].
References
| [1] | Koopmans M and Duizer E (2004). Foodborne viruses: an emerging problem. Int. J. Food Microbiol, 90: 23-41. |
| [2] | Ritter AC and Tondo EC (2014). Foodborne illnesses in Brazil: control measures for 2014 FIFA World Cup travellers. J. Infect. Dev. Ctries, 8: 254-257. |
| [3] | Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. (2011). Foodborne illness acquired in the United States-Major pathogens. Emerg Infect Dis, 17(1): 7–15. |
| [4] | Ciocca, M (2000). Clinical course and consequences of hepatitis A infection. Vaccine, 18: S71-S74. |
| [5] | Anonymous (2013). Microbiology of food and animal feed: Horizontal method for detection of Hepatitis A virus and Norovirus in food using real-time RT-PCR: Part 2: Method for qualitative detection Geneva: ISO/TS 15216-2. |
| [6] | Bidawid S, Farber JM and Sattar SA (2000). Rapid concentration and detection of Hepatitis A virus from lettuce and strawberries. Journal of Virological Methods, 88: 175-185. |
| [7] | Bruni R, Taffon S, Equestre M, Chionne P, Madonna E, Rizzo C, et al. (2016). Key role of sequencing to trace Hepatitis A viruses circulating in Italy during a large multi-country European foodborne outbreak in 2013. PLoS ONE, 11(2): e0149642. |
| [8] | Fan D, Wang C, Zhang Y, Li X and Wang X (2006). The seroepidemiological investigation of HAV among the population aged from 1 to 15 years in HAV outbreak area in Yili Prefecture. Disease Surveill, 21(10): 526–528. |
| [9] | CDC. Date, (2011). Norovirus: Technical Fact Sheet. Available at: http://www.cdc.gov/ncidod/dvrd/revb/gastro/Norovirus-factsheet.htm. Accessed June 6, 2011. |
| [10] | Rackoff LA, Bok K, Green KY and Kapikian AZ (2013). Epidemiology and evolution of rotaviruses and Noroviruses from an archival WHO Global Study in Children (1976-1979) with implications for vaccine design. PLoS One, 8: e59394. |
| [11] | da Silva Soares L, de Fátima dos Santos Guerra S, do Socorro Lima de Oliveira A, da Silva dos Santos F, de Fátima Costa de Menezes EM and Mascarenhas JP (2014). Diversity of rotavirus strains circulating in Northern Brazil after introduction of a rotavirus vaccine: high prevalence of G3P[6] genotype. J. Med. Virol, 86: 1065-1072. |
| [12] | DeWaal CS and Bhuiya F (2007). Outbreaks by the numbers: fruits and vegetables 1990-2005. In International Association for Food Protection Poster Presentation P3-03, Orlando, Florida. |
| [13] | Croci L, Dubois E, Cook N, de Medici D, Schultz AC, China B, et al. (2008). Current methods for extraction and concentration of enteric viruses from fresh fruit and vegetables: towards international standards. Food Analytical Methods, 1(2): 73–84. |
| [14] | Katzenelson E, Fattal B and Hostovesky T (1976). Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Applied and Environmental Microbiology, 32(4): 838-839. |
| [15] | Mäde D, Trübner K, Neubert E, Höhne M and Johne R (2013). Detection and typing of Norovirus from frozen strawberries involved in a large-scale gastroenteritis outbreak in Germany. Food and Environmental Virology, 5(3): 162–168. |
| [16] | Lee AR, Lee SG, Kang LH, Jheong WH and Paik SY (2013). Full length genomic sequence of subgenotype IIIA Hepatitis A virus isolate in Republic of Korea. BioMed Research International, 2013: 11. |
| [17] | Lew JF, Petric M, Kapikian AZ, Jiang X, Estes MK and Green KY (1994). Identification of minireovirus as a Norwalk-like virus in pediatric patients with gastroenteritis. J. Virol, 68(5): 3391–3396. |
| [18] | Saito H, Saito S, Kamada K, Harata S, Sato H, Morita M and Miyajima Y (1998). Application of RT-PCR designed from the sequence of the local SRSV strain to the screening in viral gastroenteritis outbreaks. Microbiol. Immunol, 42(6): 439–446. |
| [19] | Kojima S, Kageyama T, Fukuda S, Hoshino F, Shinohara M, Uchida K, et al. (2002). Genogroup-specific PCR primer for detection of Norwalk-like viruses. J. Virol. Methods, 100: 107-114. |
| [20] | Snedecor GW and Chochran WG (1982). Statistical Methods. 7th ed. Iowa State Univ. Press. Iowa, USA. |
| [21] | CDC (2003). Foodborne transmission of Hepatitis A---Massachusetts, 2001. Morbidity and Mortality Weekly Report, Morbidity and Mortality Weekly 52: 565-567. |
| [22] | Kamel AH, Ali MA, El-Nady HG, Aho S, Pothier P and Belliot G (2010). Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. Journal of Applied Microbiology, 108: 1620-1629. |
| [23] | El-Senousy WM, Costafreda MI, Pintó RM and Bosch A (2013). Method validation for Norovirus detection in naturally contaminated irrigation water and fresh produce. International Journal of Food Microbiology, 167: 74-79. |
| [24] | El-Senousy WM, Sidkey NM, Abu Senna AS, Abed NN and Hasan SF (2013). Prevalence of rotaviruses and Noroviruses in ground water of some rural areas in El-Giza Governorate, Egypt. The new Egyptian Journal of Medicine, 48(3): 18-25. |
| [25] | El-Senousy WM, El-Gamal MS, Mousa AA, El-Hawary SE and Fathi MN (2014). Prevalence of Noroviruses among detected enteric viruses in Egyptian aquatic environment. World Applied Sciences Journal, 32(11): 2186-2205. |
| [26] | El-Dougdoug K, Nasr-Eldin MA, Esawy HS, Amer MM and Abd Elrhiem MM (2017). Monitoring of foodborne Hepatitis A virus outbreaks in the fresh foods. Egyptian Journal of Botany, 57: 73-83. |
| [27] | Guevremont. E, Brassard J, Houde A, Simard C and Trottier YL (2006). Development of an extraction and concentration procedure and comparison of RT-PCR primer systems for the detection of Hepatitis A virus and Norovirus GII in green onions. J. Virol. Methods, 134(1-2): 130-135. |
| [28] | Dubois E, Agier C, Traoré O, Hennechart C, Merle G, Crucière C and Laveran H (2002). Modified concentration method for the detection of enteric viruses on fruits and vegetables by reverse transcriptase-polymerase chain reaction or cell culture. J. Food Protect, 65(12): 1962-1969. |
| [29] | Everis L (2004). Risks of pathogens in ready-to-eat fruits, vegetables, and salads through the production process. Chippin Campden, UK: Review no. 44, Campden and Chorleywood Food Research Association Group. |
| [30] | Baert L, Debevere J and Uyttendaele M (2009). The efficacy of preservation methods to inactivate foodborne viruses. Int J Food Microbiol, 131: 83–94. |
| [31] | Kukavica-Ibrulj I, Darveau A, Jean J and Fliss I (2004). Hepatitis A virus attachment to agri-food surfaces using immunological, virological and thermodynamic assays. J. Appl. Microbiol, 97: 923-934. |
| [32] | Bidawid S, Malik N, Adegbunrin O, Sattar SA and Farber JM (2004). Norovirus cross-contamination during food handling and interruption of virus transfer by hand antisepsis: Experiments with feline calicivirus as a surrogate. J. Food Prot, 67: 103-109. |
| [33] | van Voorthuizen EM, Ashbolt NJ and Schäfer AI (2001). Role of hydrophobic and electrostatic interactions for initial enteric virus retention by MF membranes. J Membrane Sci, 194: 69-79. |
| [34] | Vega E, Garland J and Pillai SD (2008). Electrostatic forces control nonspecific virus attachment to lettuce. J. Food Prot, 71: 522-529. |
| [35] | Vega E, Smith J, Garland J, Matos A and Pillai SD (2005). Variability of virus attachment patterns to butterhead lettuce. J. Food Prot, 68: 2112-2117. |
| [36] | Wang H, Zheng H, Cao J, Zhou W, Yi Y, Jia Z, et al. (2013). Genetic diversity of Hepatitis A virus in China: VP3-VP1-2A genes and evidence of Quasispecies distribution in the isolates. PLoS ONE, 8(9), e74752. |
| [37] | Richards GP (2001). Food-borne pathogens. Enteric virus contamination of foods through industrial prarctices: a primer on intervention strategies. Journal of Industrial Microbiology and Biotechnology, 27: 117-125. |
| [38] | Harris LJ, Farber JN, Beuchat LR, Parish ME, Suslow TV, Garrett EH and Busta, FF (2003). Outbreaks associated with fresh produce: incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Comprehensive reviews in Food Science and Food Safety, 2: 78-141. |
| [39] | Seymour IJ and Appleton H (2001). Foodborne viruses and fresh produce. Journal of Applied Microbiology, 91: 759-773. |
| [40] | Dentinger CM, Bower WA, Nainan OV, Cotter SM, Myers G, Dubusky LM, Fowler S, Salehi EDP and Bell BP (2001). An outbreak of hepatitis A associated with green onions. The Journal of Infectious Diseases, 183: 1273-1276. |
| [41] | Sharps CP, Kotwal G and Cannon JL (2012). Human norovirus transfer to stainless steel and small fruits during handling. J Food Prot, 75: 1437-1446. |
| [42] | Escudero BI, Rawsthorne H, Gensel C and Jaykus LA (2012). Persistence and transferability of Noroviruses on and between common surfaces and foods. J Food Prot, 75: 927-935. |
| [43] | Doyle MP and Erickson MC (2008). Summer meeting 2007- the problems with fresh produce: an overview. J. Appl. Microbiol, 105: 317-330. |
| [44] | Hassan AN and Frank JF (2003). Influence of surfactant hydrophobicity on the detachment of Escherichia coli O157:H7 from lettuce. Int. J. Food Microbiol, 87(1–2): 145–152. |
| [45] | Lodder WJ and de Roda Husman AM (2005). Presence of Noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl Environ Microbiol, 71: 1453-1461. |
| [46] | Pusch, D, Oh DY, Wolf S, Dumke R, Schroter-Bobsin U, Hohne M, Roske I and Schreier E (2005). Detection of enteric viruses and bacterial indicators in German environmental waters. Arch Virol, 150: 929-947. |
| [47] | van den Berg H, Lodder W, van der Poel W, Vennema H and de Roda Husman AM (2005). Genetic diversity of Noroviruses in raw and treated sewage water. Res Microbiol, 156: 532-540. |
| [48] | Schijven JF (2001). Virus removal from groundwater by soil passage; modeling, field and laboratory experiments. Ph.D Dissertation. Technical University, Delft, The Netherlands. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML