-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2018; 8(3): 69-73
doi:10.5923/j.microbiology.20180803.03

Light Emitting Diode (LED) based Fluorescent Microscopy versus Bright Field Microscopy for the Diagnosis of Tuberculosis from Extrapulmonary and Non-sputum Pulmonary Samples
Aravind Adarsh, Johny Asir G., Reba Kanungo
Department of Microbiology, Pondicherry Institute of Medical Sciences, Pondicherry, India
Correspondence to: Johny Asir G., Department of Microbiology, Pondicherry Institute of Medical Sciences, Pondicherry, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

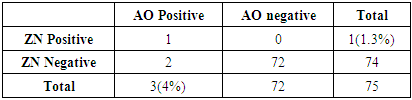
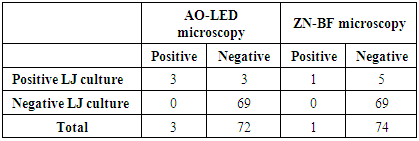
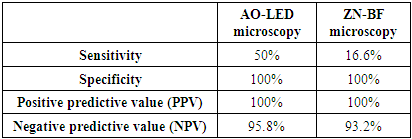
Microscopy is the mainstay in the diagnosis of tuberculosis (TB) in resource poor settings. Usefulness of Auramine-O staining and LED (AO-LED) microscopy for the diagnosis of tuberculosis from extrapulmonary and non-sputum pulmonary samples was evaluated. A prospective study was performed on 75 extrapulmonary samples like CSF, Urine, Pleural fluid and non-sputum pulmonary samples like BAL. Smears were stained with auramine-O and Ziehl-Neelsen (ZN) stains and viewed under LED microscopy and bright field(BF) microscopy respectively. Solid culture (Lowenstein-Jensen medium) was used as a reference standard. Out of the 75 specimen collected, 24% of them were CSF followed by BAL specimen (23%) and pleural fluid (13%). Three (4%) were positive by AO-LED microscopy whereas only one (1%) was positive by ZN-BF microscopy. The percentage of TB positivity among the three different methods used, revealed a high yield by culture (8%), followed by AO-LED (4%) and ZN-BF (1.3%) methods. AO-LED microscopy had a better sensitivity of 50% Vs 16.6% in comparison to ZN-BF microscopy. Both AO-LED and ZN-BF microscopy methods had a specificity and positive predictive value of 100%. Both the techniques had a negative predictive value of 95.8% and 93.2% respectively. We found a higher smear positivity rate with AO-LED in comparison with ZN-BF microscopy. The AO-LED microscopy was found to be more sensitive than the ZN-BF (50% Vs 16.6%). We report that it would be prudent to use AO-LED microscopy for non-sputum samples like BAL replacing the current practice of ZN-BF microscopy for the diagnosis of smear negative pulmonary tuberculosis.
Keywords: Auramine-O, Ziehl-Neelsen, BAL, Tuberculosis, LED microscope
Cite this paper: Aravind Adarsh, Johny Asir G., Reba Kanungo, Light Emitting Diode (LED) based Fluorescent Microscopy versus Bright Field Microscopy for the Diagnosis of Tuberculosis from Extrapulmonary and Non-sputum Pulmonary Samples, Journal of Microbiology Research, Vol. 8 No. 3, 2018, pp. 69-73. doi: 10.5923/j.microbiology.20180803.03.
Article Outline
1. Introduction
- Tuberculosis is a major cause of morbidity and mortality in the developing world. It is the leading cause of death amounting to 1.3million deaths worldwide, due to a single infectious agent ranking above HIV/AIDS. Tuberculosis (TB) patients in India accounts for nearly 1/4th of the total global burden of TB. The total estimated incidence of TB in India in the year 2016 accounted to nearly 2.28 million cases out of a global incidence of 10.4 million [1]. All these signify the importance of early diagnosis and treatment of tuberculosis as most of these deaths could have been prevented. In the advent of the 21st century, newer and rapid molecular diagnostic methods like CB-NAAT (cartridge based nucleic acid amplification) and LPA (Line probe assay) methods took the lead in the diagnosis of tuberculosis. However, in developing countries like India the use of these expensive methods pose an economic burden, with the national budget for TB prevention and care doubling from 280 million USD in 2016 to 525 million USD in 2017 [1]. This financial limitation would render expensive molecular methods unavailable at the point of care centers, and leaves only the economically feasible and time tested microscopy and culture as the solution. Though culture is described as the gold standard, it requires long incubation periods like 1-3 weeks and 3-8 weeks for liquid and solid cultures respectively [2]. Apart from the long turnaround time for obtaining results, culture is performed only at specialized laboratories particularly the reference laboratories, where specialized equipment and trained staff are available [3]. On the contrary, microscopy is rapid, less labour intensive, does not require specialized equipment and are very much available and suitable for primary health centers [4]. In a resource poor setting, the diagnosis of pulmonary and extrapulmonary tuberculosis relies on identification of acid fast bacilli by the Ziehl-Neelsen (ZN) staining and bight field microscopy. The ZN microscopy is highly specific but lacks sensitivity (20-80%) [4].Of late the Light-emitting diode (LED) based fluorescent microscope using the auramine-O staining has been found to have an increased sensitivity by 10% [5]. The World Health Organization (WHO) and the Revised National Tuberculosis Control Program (RNTCP) have recommended the use of Auramine-O staining and Light-emitting diode (LED) microscopy for the diagnosis of pulmonary tuberculosis [6, 7]. Previous studies reporting the usefulness Auramine-O LED fluorescence (AO-LED) microscopy in comparison to ZN bright field microscopy (ZN-BF) have been done exclusively for pulmonary TB suspects and on sputum samples [8-10]. Diagnosis of TB from extrapulmonary sites and non-sputum pulmonary specimen like bronchoalveolar lavage (BAL) and gastric aspirates still relies on ZN microscopy. There is paucity in data on evaluating Auramine-O LED microscopy for the diagnosis of extrapulmonary tuberculosis. Hence, we in this study intend to evaluate the usefulness of AO-LED microscopy for the diagnosis of tuberculosis from extrapulmonary and non-sputum pulmonary samples.
2. Materials & Methods
2.1. Setting
- This prospective study was performed during the period of August 2016 to November 2016 at the designated microcopy center (DMC) of the department of Microbiology, Pondicherry Institute of Medical Sciences, a tertiary care center located in the Union territory of Pondicherry, India. The study was approved by the Institutional research and ethics committee.
2.2. Specimen Collection
- Seventy five extrapulmonary samples like cerebro-spinal fluid (CSF), peritoneal /pleural fluid, urine, tissue, ascitic fluid, bone marrow from suspected TB patients that were received in the department of microbiology were included in the study. Non-sputum pulmonary samples like gastric aspirates and Bronchoalveolar lavage (BAL) was also included in the study.
2.3. Preparation of Stains
- Ziehl-Neelsen stain and the Auramine-O stain reagents were prepared according to the standard recommendations as per the RNTCP guidelines as described earlier [11, 12]. The reagents were prepared and stored in dark bottle and stored at room temperature till further use.
2.4. Preparation of Smear and Staining
- All specimens were centrifuged at 2000 RPM for 10 minutes. With an inoculating loop the deposit were smeared over 2 grease-free clean glass slides. The slides were numbered appropriately and the details were blinded to the microscopist. The Smears were heat fixed and stained according to the standard RNTCP guidelines as described earlier [11, 12]. Staining was done in duplicates using the Ziehl-Neelsen staining (Conc.carbol fuchsin, 25% sulphuric acid and 0.1% methylene blue) for bright field microscopy and Auramine-O staining (Auramine-phenol, 1% acid-alcohol and 0.1% potassium permanganate) for LED microscopy.
2.5. Microscopy
- The Ziehl-Neelson stained smears were then viewed under Olympus-CH20i bright field microscope (Magnification-1000X) and Auramine-O smears under Carl Zeiss-Primo Star iLED microscope (magnification-400X).
2.6. Culture
- After decontamination and concentration by modified Petroff’s method using 4% NaOH, the specimen was inoculated onto two sets of Lowenstein-Jensen (LJ) medium and one LJ slope with Para-nitrobenzoic acid (500µg/ml). The inoculated media were incubated at 37°C and observed weekly for visible growth of colonies for upto 8 weeks. When macroscopic growth was observed, colonies were picked up and confirmed by ZN staining.
2.7. Statistical Analysis
- All the parameters were analysed descriptively using statistical methods. The data obtained based on count were expressed as either numbers or percentages (%). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of Zeihl-Neelsen-Bright field (ZN-BF) microscopy and Auramine-O-LED (AO-LED) microscopy were determined, comparing it with culture as the gold standard.
3. Results
- A total of 75 extrapulmonary and non-sputum pulmonary samples were collected from tuberculosis suspects during the study period. Majority of the samples 44(58.6%) were from males followed by 31(41.3%) from females. The age of tuberculosis suspects ranged from 4 months to 77 years. Majority of the patients 37(49.3%) were from the middle aged (18-59) group followed by elderly 30(40%) and 8(10.6%) in the pediatric age group.Out of the 75 specimen collected, 24% of them were CSF followed by BAL specimen which was 23%, followed by pleural fluid (13%). (Figure 1).
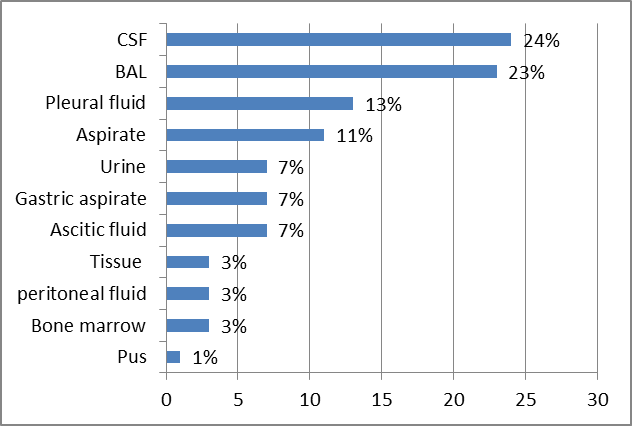 | Figure 1. Distribution of different specimen received from tuberculosis suspects |
|
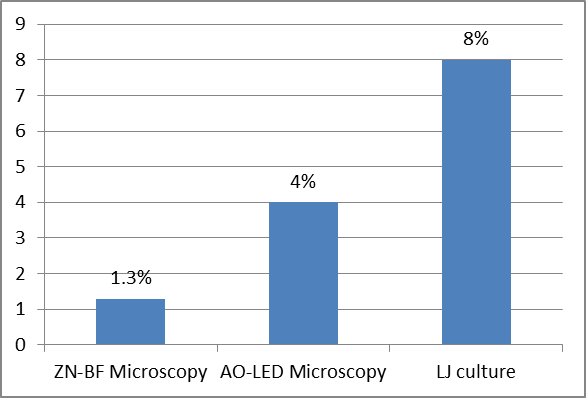 | Figure 2. Percentage of positivity of the three diagnostic methods performed among TB suspects |
|
|
4. Discussion
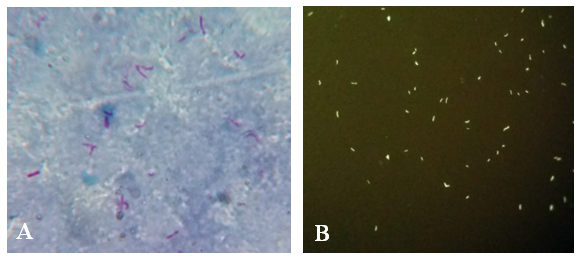
- Microscopy is the mainstay in the diagnosis of pulmonary and extrapulmonary tuberculosis in the resource poor setting. Auramine-O staining and LED microscopy has been extensively evaluated and is currently in use for sputum microscopy. However, extrapulmonary samples and non-sputum samples like BAL are still being stained by the conventional ZN stain and viewed by the bright field microscopy which has a very low sensitivity. The present study evaluated the role of AO-LED microscopy in the diagnosis of TB from extrapulmonary and non-sputum samples like BAL.A vast majority of TB suspects in our study were males 44(58%), which is in consensus with a study done by Austin et al, which could be attributed to the better health seeking behaviour among male population [13]. Most of them (49%) also belonged to the middle age group. TB meningitis was the most suspected clinical condition in our study, hence CSF samples constituted the highest number of samples followed by BAL.We found a higher smear positivity rate with AO-LED in comparison with ZN-BF microscopy, in concurrence with previous studies [13, 14]. This can be explained by the fact that fluorescent colour offers a better contrast against a black background in AO-LED than pink against a blue background as in ZN-BF (Figure 3). Moreover, AO-LED microscopy uses a magnification of 400X covering a larger field of the slide. ZN-BF uses a bigger magnification of 1000X and covers a smaller field thereby increasing the probability of missing the bacilli.
5. Conclusions
- This study evidently highlights the importance and advantage of the AO-LED microscopy for diagnosis of pulmonary tuberculosis from non-sputum pulmonary samples like BAL. The increased and superior sensitivity of AO-LED over ZN-BF (50% Vs 16.6%) and better negative predictive value (95.8% vs 93.2%) proves the above statement. Our study also showed the improved diagnostic value of AO-LED microscopy in detecting tuberculosis in sputum smear negative patients. The current practice of ZN-BF microscopy for Staining BAL samples had missed the diagnosis of TB in one of our sputum smear negative patients. However, AO-LED microscopy picked up the bacilli in the same patient. The fluorochrome stain (Auramine-O) and the LED microscope is far more superior and efficient in detecting acid fast bacilli from BAL samples due to the better affinity of the stain to the bacilli and moreover, larger area can be viewed in a single field. This may be further accentuated by studies using larger sample size.In conclusion, we report that it would be prudent to use AO-LED microscopy for non-sputum samples like BAL replacing the current practice of ZN-BF microscopy for the diagnosis of smear negative pulmonary tuberculosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML