-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2018; 8(3): 60-68
doi:10.5923/j.microbiology.20180803.02

In Vitro Evaluation of the Resistance of Seven Isolates of Colletotrichum acutatum to Thiram and Copper
Rababe Es-Soufi1, Mohammed L’bachir El Kbiach1, Tomader Errabii1, Rabah Saidi1, 2, Alain Badoc3, Ludovic Chaveriat4, Patrick Martin4, Ahmed Lamarti1
1Laboratory of Plant Biotechnology, Biology Department, Faculty of Sciences, Abdelmalek Essaadi University, Tetouan, Morocco
2Department of Matter and Life Sciences, High Normal School, Martil, Morocco
3Axe MIB, Univ. Bordeaux, Unité de recherche Œnologie, EA 4577, USC 1366 INRA, ISVV, 33882 Villenave-d’Ornon Cedex, France
4Univ. Artois, UniLasalle, EA7519 - Unité Transformations & Agro-ressources, F-62408, Béthune, France
Correspondence to: Rababe Es-Soufi, Laboratory of Plant Biotechnology, Biology Department, Faculty of Sciences, Abdelmalek Essaadi University, Tetouan, Morocco.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Seven Isolates of Colletotrichum acutatum have been collected from strawberries attacked with anthracnose in the Loukous territory, Morocco. The study of the effect in vitro of 3 fungicides with Thiram as active substance and another one with copper on seven isolates of C. acutatum was studied. Thiram has a potent effectiveness on germination and is moderately effective on mycelial growth and sporulation. Copper has shown effectiveness on germination as variable activity in other strain’s life stage.
Keywords: Anthracnose, Strawberry, Colletotrichum acutatum, Loukous, Morocco, Fungicides, Thiram, Copper
Cite this paper: Rababe Es-Soufi, Mohammed L’bachir El Kbiach, Tomader Errabii, Rabah Saidi, Alain Badoc, Ludovic Chaveriat, Patrick Martin, Ahmed Lamarti, In Vitro Evaluation of the Resistance of Seven Isolates of Colletotrichum acutatum to Thiram and Copper, Journal of Microbiology Research, Vol. 8 No. 3, 2018, pp. 60-68. doi: 10.5923/j.microbiology.20180803.02.
Article Outline
1. Introduction
- Anthracnose is one of the most important diseases that attacks strawberry (Fragaria x ananassa) and is a limiting factor for its culture by its devastating nature, the sensitivity of the cultivars used and the low efficiency of control measures available [1-4].Several Colletotrichum species are involved in strawberry anthracnose, affecting rhizomes, stolons, petioles, leaf blade and apical meristem [5]. The incidence and severity of symptoms occur due to the variability among the isolates of pathogens which prevail in a given region and its interaction with the cultivars used, that give the disease very complex [2-8]. Colletotrichum acutatum is one of the most frequently reported species responsible for anthracnose on many host plants across the world, including legumes, small fruits and perennial crops, in more than 40 plant families [9-12]. This fungus has a significant economic impact, particularly on strawberry (Fragaria x ananassa) [13, 14]. It can infect all parts of the strawberry plant, leading to substantial losses in fruit production [15].The control of fungal phytopathogens to cope with some of the problems that have been observed in the clinical field related, including the selection of strains of antifungal agents-tolerant and relatively few classes of fungicides currently available and effective [16].The effective control against anthracnose involved the use of chemical control and the induction of strawberry resistance [17-20]. The intensive use of fungicides has resulted in the accumulation of toxic compounds potentially dangerous to humans and the environment, and increasing resistance of the pathogens.Since 2012, three fungicides based on Thiram (Thiramic, Basultra and Thiramchim) have been approved for the agricultural use by ONSSA Morocco (ONSSA database, approval of chemical inputs). To prevent the disease, farmers of Loukous area also use a product based on copper (Bordeaux Caffaro).The aim of this study was to assess the effect of these plant protection products on the different stages of Colletotrichum acutatum development.
2. Material and Methods
2.1. Fungal Material
- Seven isolates of C. acutatum have been used (Ca1, Ca2, Ca3, Ca4, Ca5, Ca6 and Ca7). These isolates were collected on infected strawberries in field (Fragaria x ananassa (Weston) Duchesne ex Rozier) of the region of Loukous, Morocco. Fungi were cultivated on PDA (Potato Dextrose Agar) medium for 7 to 10 days at 25°C in the dark. Successive subculturing was made up to total purification of the isolates.A spore suspension outcome of conidia was used for the study of germination. Mycelial discs of 5 mm diameter from 10-day cultures were vortexed in a tube containing 5 ml of sterile distilled water. The suspension was diluted with sterile distilled water to obtain 10 000 spores/ml by counting on a Malassez cell under optical microscope with a magnification of 400. For each of the three repetitions, new mycelial disks were used.
2.2. Fungicides Used
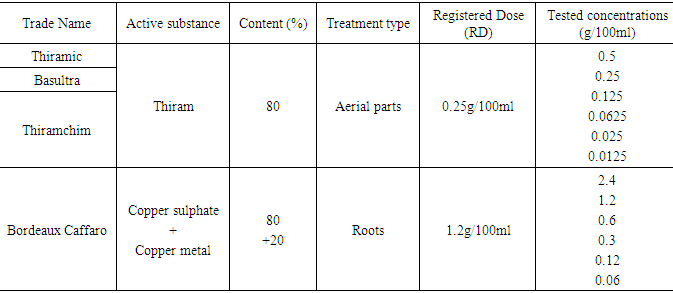
- Three fungicides, containing the same active ingredient Thiram, and a product based on copper, all used to fight strawberry anthracnose in Morocco, have been tested on the different stages of development of Colletotrichum acutatum (Table 1).
|
2.3. Effect on Mycelial Growth
- A concentrated solution has been prepared by solubilization in sterile distilled water for fungicides soluble in water (case of Bordeaux Caffaro) or in 0.5% chloroform for the other [21] (fungicides based on Thiram).Different concentrations of fungicides have been added to 90 ml of PDA. Volumes of 15 to 20 ml of medium were sunk in petri dishes of 90 mm diameter.They were inoculated by a mycelial disk from 10-day old culture. After 10-day incubation at 25°C in the dark, the growth has been assessed by average of two perpendicular diameters. Three petri dishes were used for each concentration and the test was repeated three times.
2.4. Effect on Spore Germination
- The different fungicide concentration tested have been added to the sterilized agar solution (1.5 g agar in 100 ml distilled water), and then poured at the rate of 15 ml in petri dishes of 90 mm diameter. Agar surfaces were inoculated by three drops of conidial suspension (about 20 µl/ drop) by a micropipette then spread of the suspension on petri dish containing water agar. After 24-hour incubation at 25°C in the dark, the counting of germinated conidia was realized under optical microscope on 100 conidia. Two dishes were used for each concentration and the test was repeated three times.
2.5. Effect on Sporulation
- Three mycelial disks of 5 mm diameter drawn from three petri dishes having served for the growth and aged of 10- days have been placed in a tube containing 1 ml of sterilized water. After vortexing, 20 µl of fungal suspension was diluted to 1 ml and ten counts were made with a Malassez cell. The test was repeated three times.
2.6. Calculations
- The percentages of inhibition of germination, mycelial growth and sporulation were calculated according to the following formula:PI = (Xo-Xc)/Xo×100Xo: outcome observed on the witnessXc: observed result in the medium supplemented with fungicidesThe average of the effects of the three Thiram fungicides was calculated to be compared with that of the copper to the registered dose on the different stages of development of the isolates of C. acutatum.IC50 and IC90 have been determined graphically by the linear relationship between the logarithm of the concentrations and the percentages of inhibition of germination, growth and sporulation.
3. Results
- The fungicides tested showed different degrees of effectiveness on the three life stages of C.acutatum at different concentrations.
3.1. Effect on Mycelial Growth
- The growth of all the isolates was significantly reduced by adding concentrations more than 1/10 of the registered dose of fungicides to basis of Thiram, but the addition of low concentration showed a low efficiency on mycelial growth (Figure 1).In the presence of Bordeaux Caffaro, even at the registered dose, the inhibition of mycelial growth of isolates does not exceed 60% (Figure 1).
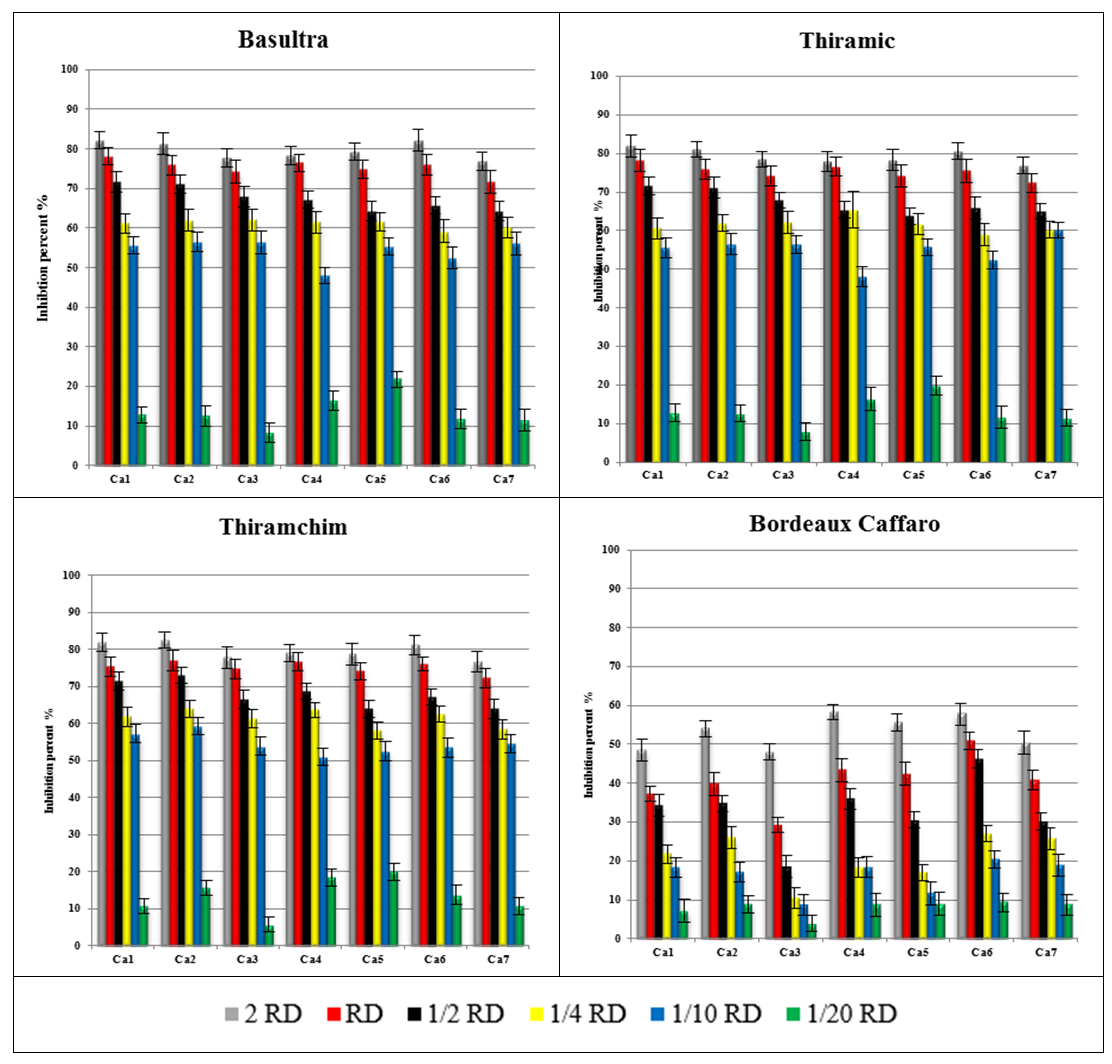 | Figure 1. Precent of inhibition of the mycelial growth of Colletotrichum acutatum isolates by six concentrations of Basultra, Thiramic and Thiramchim, and Bordeaux Caffaro |
3.2. Effect on Spore Germination
- The fungicides at basis of Thiram have a great effectiveness (100% inhibition) on the germination of all isolates to 2 RD, RD and ½ RD even the 1/20 RD of these fungicides gave us an inhibition which exceeds 60% (Figure 2) Bordeaux Caffaro completely inhibited the germination of the isolates to 2 RD and RD and remained effective has for other concentrations (Figure 2).
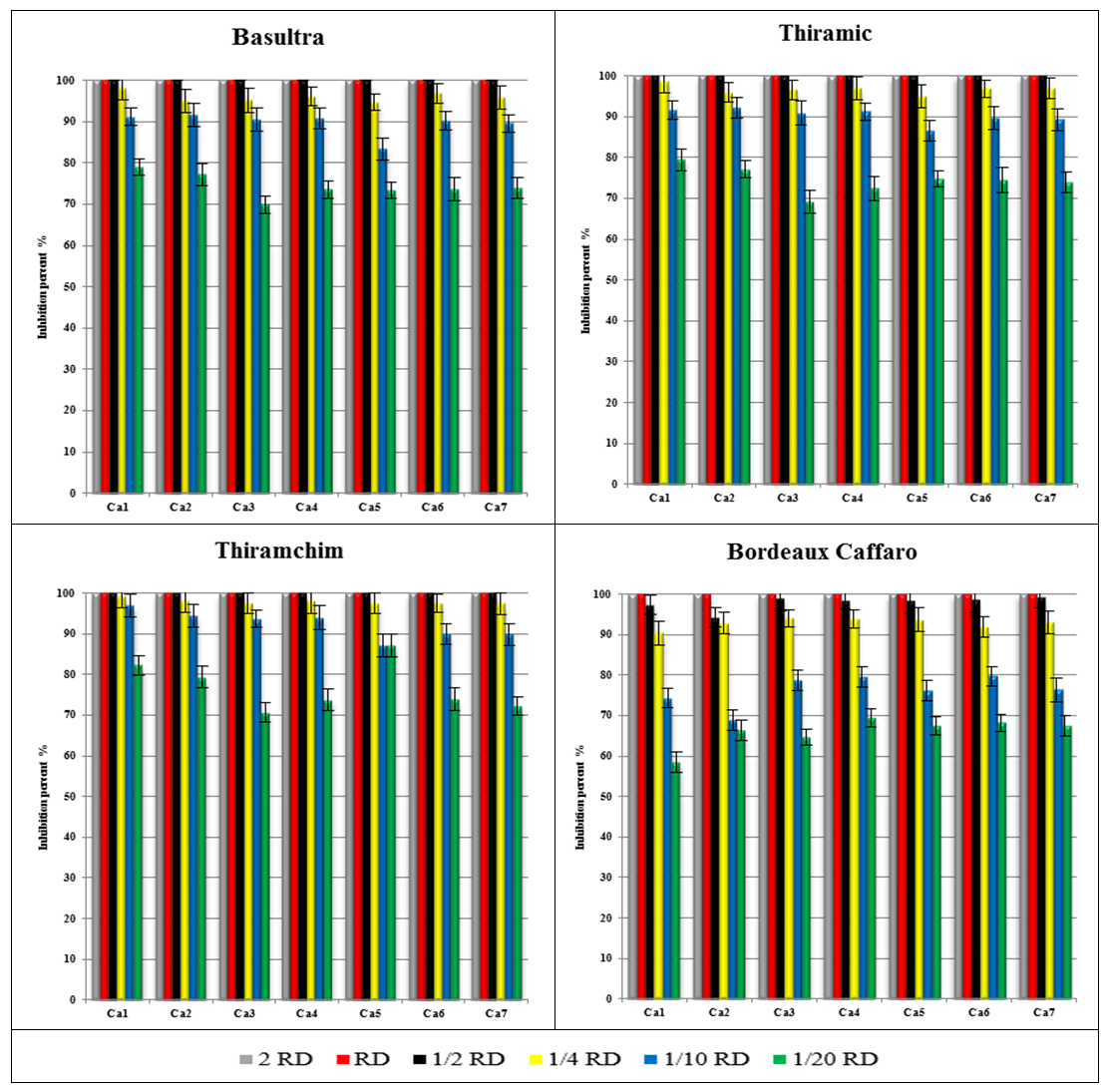 | Figure 2. Inhibition percent of six concentrations of fungicides on the germination of seven Colletotrichum acutatum isolates |
3.3. Effect on Sporulation
- Thiram is not totally effective on sporulation of all strains of C. acutatum. Inhibition is substantial at 2 RD, RD, ½ RD. The strains do not have the same sensitivity, and Ca6 is the least resistant (Figure 3).The presence of 2 RD, RD, ½ RD and ¼ RD of Bordeaux Caffaro allowed to see a great sensitivity of the Ca2 and 4, and the addition of 1/10 RD and 1/20 RD showed that the effectiveness of this fungicide decreased (Figure 3).
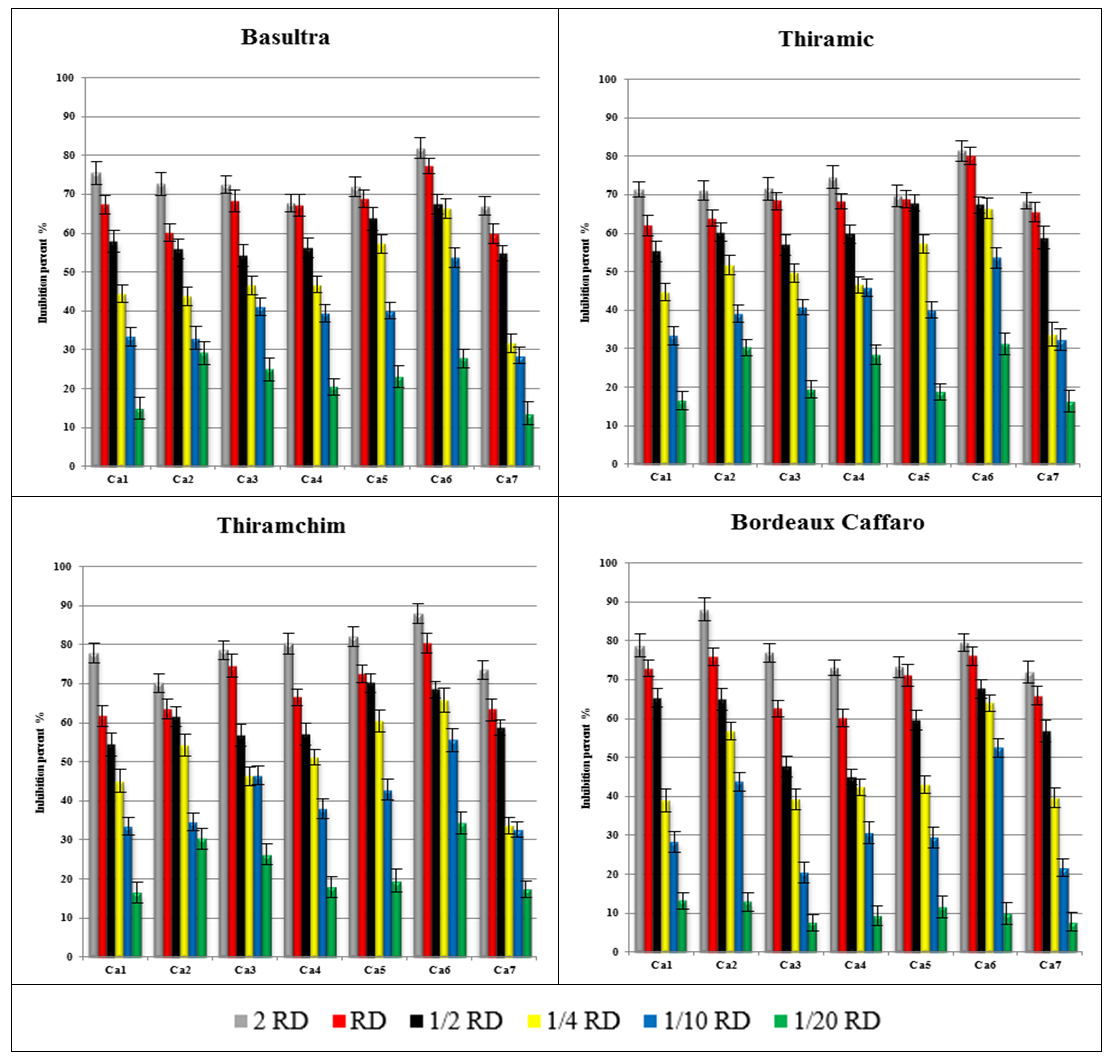 | Figure 3. Inhibition percent of six concentrations of fungicides on sporulation of seven Colletotrichum acutatum isolates |
3.4. Fungicides Inhibitory Concentration
3.4.1. On Mycelial
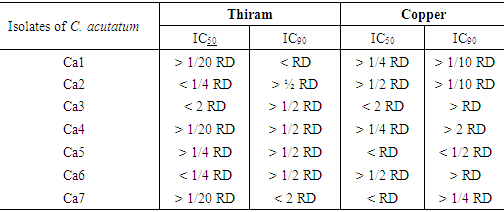
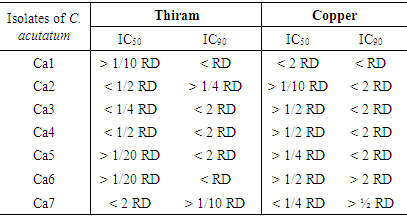
- The mycelial growth was not totally inhibited by the four fungicides. A mycelial development is observed at different degrees (Figure 4). Thiram is active on the mycelial growth of all strains and copper moderately effective (Table 2).
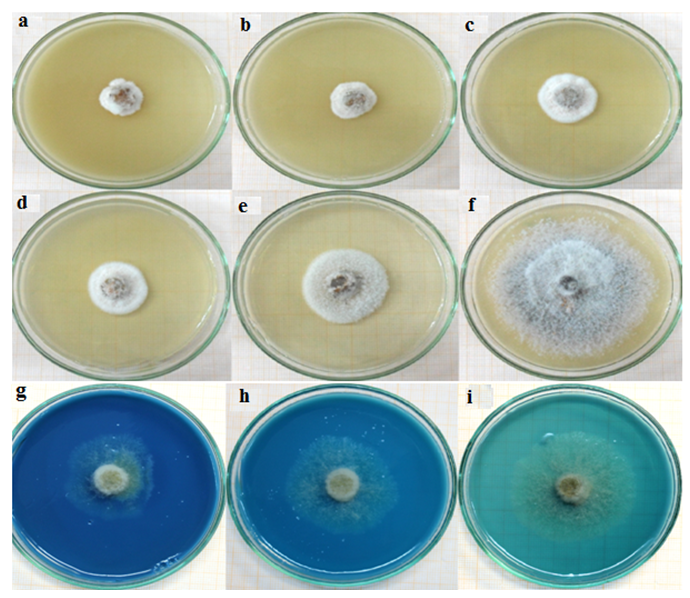 | Figure 4. Mycelial growth of strain Ca6 in the presence of Thiramic. a) 2 RD; b) RD; c) ½ RD; d) ¼ RD; e) 1/10 RD; f) 1/20 RD and Bordeaux Caffaro. g) 2 RD; h) RD; i) ½ RD |
|
3.4.2. On Germination
- Copper and Thiram are very effective to completely inhibited germination (100% inhibition) of isolates of C. acutatum to the double dose certified and to RD, even at the half dose in case of Thiram.
3.4.3. On Sporulation
- Thiram and Copper have a remarkable effect on sporulation of C. acutatum strains (Table 3).
|
3.4.4. Colletotrichum acutatum sensitivity to Thiram and Copper at RD
- The registered dose (RD) of Copper and Thiram is very effective to completely inhibited the germination, Thiram is moderately effective on mycelial growth and sporulation (Figure 5).
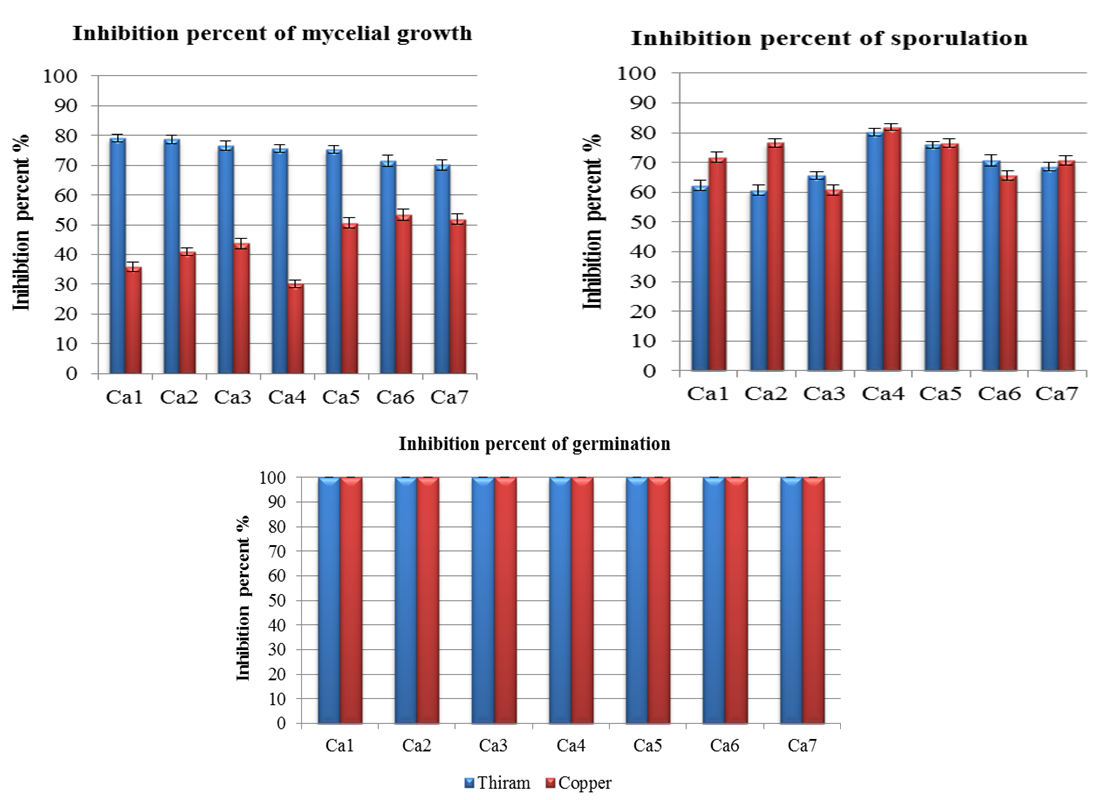 | Figure 5. Comparison of the averages of inhibition of Thiram and Copper to the registered dose on three stages of development of Colletotrichum acutatum |
4. Discussion and Conclusions
- The effect of Thiram varied according to the concentration tested and the life stages of isolates of C. acutatum. The registered dose of Thiram remaind moderately effective against the mycelial growth and sporulation of isolates and very efficient with respect to the germination of spores of the strains tested. At two times the registered dose, Thiram was effective on mycelial growth and sporulation, but it is not advised to use high concentrations against anthracnose because of a greater risk to the environment and the users. Same result has been obtained by increasing the concentration of Thiram on the development of C. acutatum strains [22].Copper at the double registered dose proved to be effective against the germination of strains of C. acutatum, but not against its growth and moderately against sporulation. This fungicide may not be able to check anthracnose and limit resistance.Other studies have been made to develop recommendations for the management of the disease; a very high efficiency has been carried out by fungicides Metiram+Piraclostrobin, Captan FL and Fludioksinil+Ciprodinil [23]. The less effective were Benomyl and Krezoksim-metil [23]. Various fungicides were evaluated for their ability to control anthracnose of strawberry caused by C. acutatum in Israel [24], and anthracnose of immature peppers in Ohio [25]. In Florida, the management of anthracnose of strawberry plant is based on the use of Captan or Thiram by a regular weekly application [26].Carbendazin, Bitertanol and Triabendazole have been found effective against C. acutatum in vivo on cultivated strawberry [27].Harp et al. [28] have found that Copper hydroxide and the other fungicides tested in their study could contribute to a successful pest management program on pepper for the control against C. acutatum. Copper hydroxide also showed an inhibitory effect on both Phytophthora capsici and Colletotrichum acutatum causing red pepper anthracnose [29]. Copper oxychloride was the best toxic fungicide inhibiting the mycelial growth in vitro at all concentrations tested [30]. Copper formulations effectively inhibited spore germination by 50% at concentrations ranging from 0.1 to 141 µg/mL, but were less effective against mycelial growth of Colletotrichum [31].Copper has been shown efficient on a wide range of yeasts and fungi such as Aspergillus (A.) carbonarius, A. fumigates, A. niger, Candida albicans, Cryptococcus neoformans, Trichoderma viride [32]. A study on the control of C. acutatum of strawberry by different fungicides have showed that Copper sulphate inhibited perfectly mycelial growth [33] and gave a remarkable effect on C. gloeosporioides [31, 34].Pesticides have been used for the well-being of human beings but show their toxicity directly or indirectly through the food chain. Pesticides are toxic compounds to all living organisms, but the effects vary from one species to another. Their excessive use caused serious damage to the ecosystem - terrestrial as well as aquatic organisms, and consequently to the flora and fauna surrounding it [35]. Pollution by heavy metals in agricultural soils is a particularly pressing concern since it affects food safety and security. Elements that are considered as micronutrients, such as Copper (Cu), are particularly relevant because plants tend to uptake them and accumulate the excess in their tissues [36].Thiram, that has also been used to treat the gray rot of strawberry grown, has no effect on the chemical composition of the strawberry plants but let residues on fruits after the treatment [37]. It was found effective against Alternaria macrospora [38]. Van Leeuwen et al. [39] have shown that Thiram metabolites can kill marine organisms. Some reports have suggested that Thiram due to its main metabolism in the liver is hepatotoxic by the imbalance and oxidative damage to the liver [40-43].A diversity of resistance has been observed is this study, so it was therefore necessary to develop alternatives to chemical control for reducing environmental and health risks. Several alternatives are promising, as effective as fungicides. A strategy should be developed that combines several of these alternatives to improve their effectiveness.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML