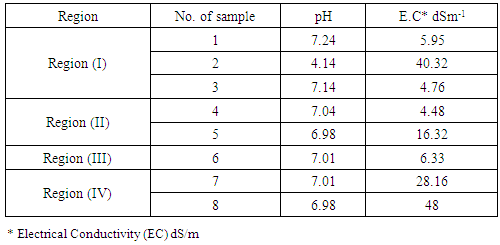
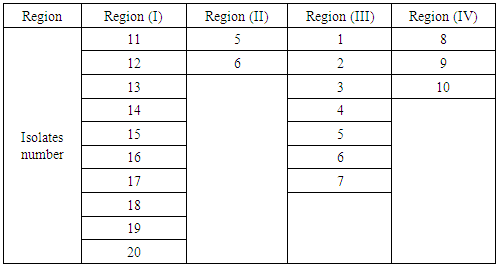
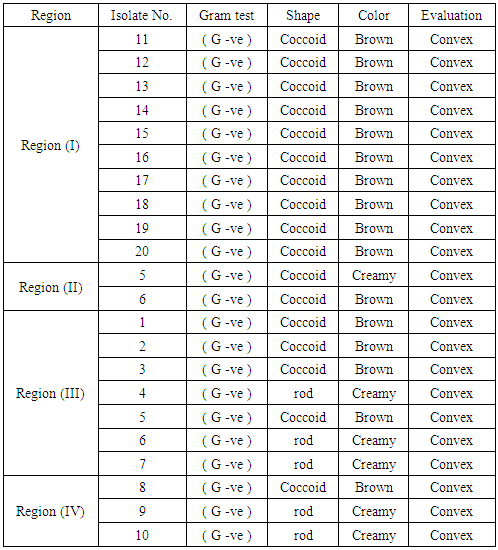
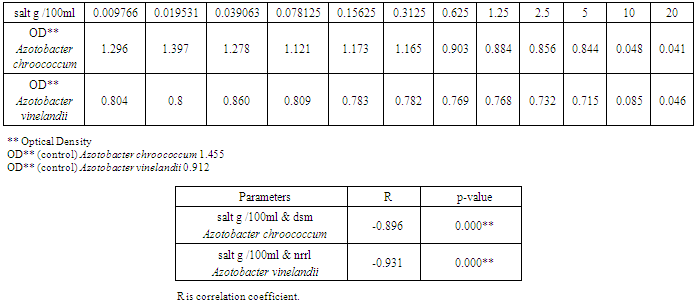
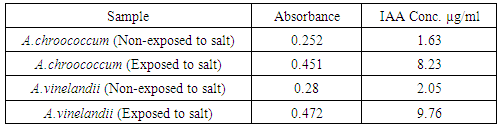
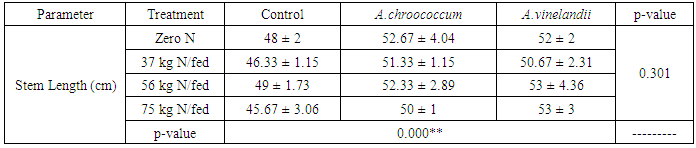
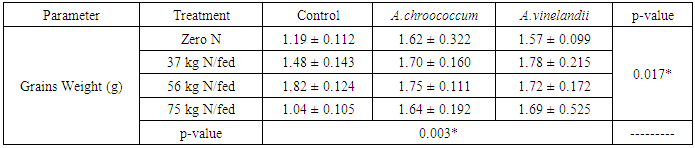
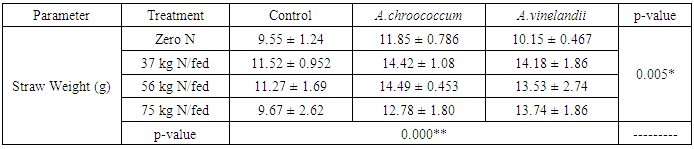
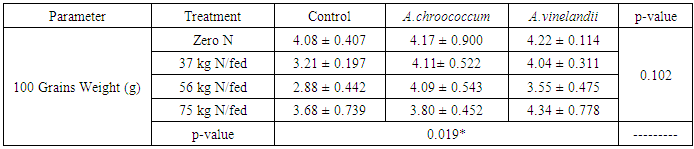
| [1] | Mer, R.K., P.K. Prajith, D.H. Pandya and A.N. Pandey, 2000. Effect of salts on germination of seeds and growth of young plants of Hordeum vulgare, Triticum aestivum, Cicer arietinum and Brassica juncea. J Agronomy and Crop Sciences, 185: 209-217. |
| [2] | Paul, D., & Nair, S. (2008). Stress adaptations in a Plant Growth Promoting Rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. Journal of Basic Microbiology, 48(5), 378-384. doi:10.1002/jobm.200700365. |
| [3] | Kayani, S.A. and M. Rahman, 1987. Salt tolerance in Corn (Zea mays L.) at the germination stage. Pak. J. Bot., 19: 9-15 |
| [4] | Rahman, M., S.A. Kayani and S. Gul, 2000. Combined effects of temperature and salinity stress on corn cv. Sunahry, Pak. J. Biological Sci., 3(9): 1459-1463. |
| [5] | Pandey, A.N. and N.K. Thakrar, 1997. Effect of chloride salinity on survival and growth of Prosopis chilensis seedlings. Trop. Ecol., 38: 145-148. |
| [6] | Hussain N, Ali A, Khan AG, Rehman OU, Tahir M (2003). Selectivity of ion absorption as nmechanism of salt tolerance I rice (variety Shaheen Basmati). Asian J plant Sci 2: 445-448. |
| [7] | Tester M, Davenport R (2003). Na+ tolerance and Na+ transport in higher plants. Ann Bot 91(5): 503–27. |
| [8] | Poljakoff-Mayber A, Somers GF, Werker E, Gallagher JL. Seeds of Koteletzkya virginica (Malvaceae): their structure, germination and salt tolerance. Am J Bot. 1994;81:54–59. doi: 10.2307/2445562. |
| [9] | Jadhav GG, Salunkhe DS, Nerkar DP, Bhadekar RK. Isolation and characterization of salt tolerant nitrogen fixing microorganisms from food. J Eur Asia Bio Sci. 2010;4:33–40. doi: 10.5053/ejobios.2010.4.0.5. |
| [10] | Abu-Zeid, M. (1988). Egyptian policies for using low quality water for irrigation. In R. Bouchet, ed. Proc. of the Cairo/Aswan seminar "Reuse of low quality water for irrigation in Mediterranean Countries". Cairo. |
| [11] | Amer MH, de Ridder NA (1989). Land drainage in Egypt. Drainage Research Institute for Land Reclamation and Improvement, Cairo. |
| [12] | FAO (2010). Database Collections (Food and Agriculture Organization, Land and Plant Nutrition Management Service.) Available from: http://www.fao.org. |
| [13] | Shirazi, M.U., S.M. Asif, B. Khanzada, M.A. Khan and A. Mohammad, (2001). Growth and ion accumulation in some wheat genotypes under NaCl stress. Pak. J. Biol. Sci., 4: 388-391. |
| [14] | Anonymous, (1999). Agricultural statistics of Pakistan: Ministry of Food, agriculture and livestock, economics wing, Islamabad, pp: 3-4. |
| [15] | FAO (2005). Database Collections (Food and Agriculture Organization, Fertilizer used by crop in Egypt.). |
| [16] | Zahir ZA, Arshad M, Frankenberger WT. Plant growth promoting rhizobacteria: applications and perspectives in agriculture. Adv Agron. 2004; 81: 97–168. doi: 10.1016/S0065-2113(03)81003-9. |
| [17] | Naz I, Bano A, Rehman B, Pervaiz S, Iqbal M, Sarwar A, Yasmin F. Potential of Azotobacter vinelandii Khsr1 as bio-inoculant. Afr J Biotechnol. 2012;11:10368–10372. |
| [18] | Sturz AV, Nowak J. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl Soil Ecol. 2000; 15: 183–190. doi: 10.1016/S0929-1393(00)00094-9. |
| [19] | Magda MA, Sabbagh SM, El-shouny WA, Ebrahim KH. Physiological response of Zea maysto NaCl stress with respect to Azotobacter chroococcum and Streptomyces niveus. Pakistan J Biol Sci. 2003;6:2073–2080. doi: 10.3923/pjbs.2003.2073.2080. |
| [20] | Wu CH, Bernard SM, Anderson GL, Chen W (2009). Developing microbe interactions for applications in plant growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microbiol Biotechnol 2: 428–440. |
| [21] | Sindhu SS, Dua S, Verma MK, Khandelwal A (2010). Growth promotion of legumes by inoculation of rhizosphere bacteria. In: Khan MS, Zaidi A, Musarrat J (eds) Microbes for legume improvement. Springer-Wien, NewYork, pp 195–235. |
| [22] | Muhammad Arshadullah, Syed Ishtiaq Hyder, Imdad Ali Mahmood, Tariq Sultan and Sadiq Naveed. (2017). Mitigation of salt stress in wheat plant (Triticum aestivum) by plant growth promoting rhizobacteria for ACC deaminase. Int. J. Adv. Res. Biol. Sci. 4(6): 41-46. |
| [23] | Rahim Nosrati., Parviz Owlia., Horieh Saderi., Iraj Rasooli., Mohammad Ali Malboobi, (2014). Iran J Microbiol,6(4): 285-295. |
| [24] | Sartaj A Wani., Subash Chand and Tahir Ali, (2013). Current Agriculture Research Journal, 1(1): 35-38. |
| [25] | Gurikar C., Naik M.K., Sreenivasa M.Y. (2016). Azotobacter: PGPR Activities with Special Reference to Effect of Pesticides and Biodegradation. In: Singh D., Singh H., Prabha R. (eds) Microbial Inoculants in Sustainable Agricultural Productivity. Springer, New Delhi. |
| [26] | Silini, A., Cherif-Silini, H., & Yahiaoui, B. (2016). Growing varieties durum wheat (Triticum durum) in response to the effect of osmolytes and inoculation by Azotobacter chroococcum under salt stress. African Journal of Microbiology Research, 10(12), 387-399. |
| [27] | Richards, L. A. (ed.) (1954). “Diagnosis and improvement of saline and alkaline soils” U.S.D.A. Agric, Handbook No. 60. |
| [28] | Vancura V and J Macura. (1960). Indole derivatives in Azotobacter cultures. Fol. Microbiol. 5: 293-298. |
| [29] | Dobereiner, J. (1978). Influence environmental factors on the occurance of Spirillum lipoferum in soil and roots. In: Granhall U (Ed), Environmental role of nitrogen-fixing bleu-green algae and asymbiotic bacteria. Ecological Bulletins, 26, Stockholm: Swedish Nature, p. 343-352. |
| [30] | Brenner, D.J., Krieg, N.R., Staley, J.T. and Garrity, G.M. (2005). Bergey’s manual of Systematic Bacteriology 2nd Ed., Vol. 2, Part (A), The Proteobacteria, Introductory Essays, New York, Springer. |
| [31] | Carozzi, N.B., Kramer, V.C., Warren, G.W., Evola, S. and Koziel, M.G. (1991). Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl. environ. Microbiol, 57, 306-3057. |
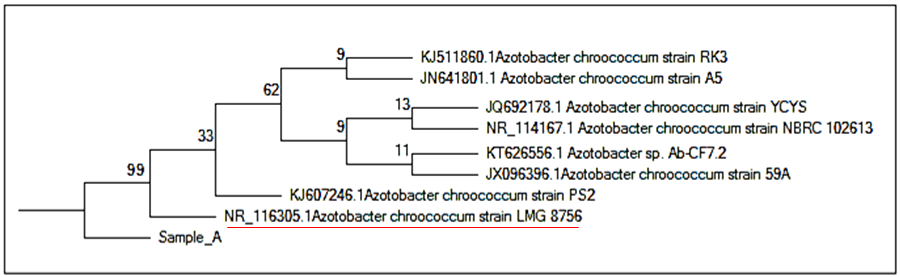
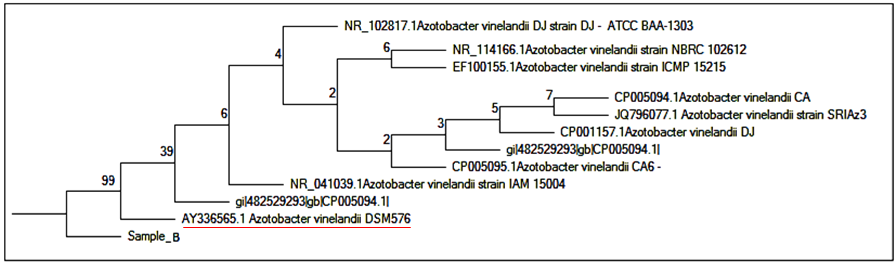
| [32] | Saitou N, Nei M (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. |
| [33] | Joseph G Pigeon (2012). Statistics for Experimenters: Design, Innovation.and. Discovery, Technometrics, 48: 2, 303-304, DOI: 10.1198/tech.2006.s379. |
| [34] | Saville D.J., Wood G.R. (1991). Split Plot Design. In: Statistical Methods: The Geometric Approach. Springer Texts in Statistics. Springer, New York, NY. |
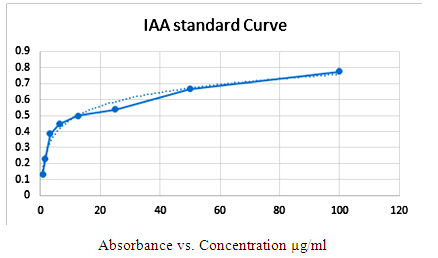
| [35] | Pilet PE, Chollet R. (1970). Sur le dosage colorimetrique de l’acide indolylacetique. C R Acad Sci Ser D. 1970; 271: 1675–1678. |
| [36] | Miller, R.W. and R.L. Donahue, (1995). Soils in our environment, 7th Ed. Prentice Hall Inc., Englewood Cliffs, New Jersey. 649p. Available from: agris.fao.org (http://www.fao.org/docrep/x5871e/x5871e04.htm). |
| [37] | Egamberdieva, D. Acta Physiol Plant (2009) 31: 861. https://doi.org/10.1007/s11738-009-0297-0. |
| [38] | Singh H, Dara BL (1971). Influence of presoaking of seeds with gibberellin and auxins on growth and yield attributes of wheat (Triticum aestivum L.) under high salinity, sodiumadsorption ratio and boron levels. Indian J Agric Sci 41: 998–1003. |
| [39] | Datta KS, Varma SK, Angrish R, Kumar B, Kumari P (1998). Alleviation of salt stress by plant growth regulators in Triticum aestivum L. Biol Plant 40:269–275. doi: 10.1023/A:1001076805595. |
| [40] | Sastry EVD, Shekhawa KS (2001). Alleviatory effect of GA3 on the effect of salt at seedling stage in wheat (Triticum aestivum). Indian J Agric Res 35: 226–231. |
| [41] | Afzal I, Basra S, Iqbal A (2005). The effect of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J Stress Physiol Biochem 1(1): 6–14. |
| [42] | Khan MA, Weber DJ (1986). Factors influencing seed germination in Salicornia pacifica var utahensis. Am J Bot 73:1163–1167. doi: 10.2307/2443795. |
| [43] | Gul B, Khan MA, Weber DJ (2000). Alleviation salinity and dark-enforced dormancy in Allenrolfea occidentalis seeds under various thermoperiods. Aust J Bot 48:745–752. doi: 10.1071/BT99069. |
| [44] | Khan MA, Gul B, Weber DJ (2001). Seed germination characteristics of Halogeton glomeratus. Can J Bot 79: 1189–1194. doi: 10.1139/cjb-79-10-1189. |
| [45] | Khan MA, Gul B, Weber DJ (2004). Action of plant growth regulators and salinity on seed germination of Ceratoides lanata. Can J Bot 82:37–42. doi: 10.1139/b03-140. |
| [46] | Kabar K (1987). Alleviation of salinity stress by plant growth regulators on seed germination. J Plant Physiol 128:179–183. |
| [47] | Sakhabutdinova AR, Fatkhutdinova DR, Bezrukova MV, Shakirova FM (2003). Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg J Plant Physiol 314–31. |
| [48] | Nagargade M., Tyagi V., Singh M.K. (2018). Plant Growth-Promoting Rhizobacteria: A Biological Approach Toward the Production of Sustainable Agriculture. In: Meena V. (eds) Role of Rhizospheric Microbes in Soil. Springer, Singapore. |
| [49] | Saia S, Rappa V, Ruisi P, et al. (2015). Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front Plant Sci 6: 815. |
| [50] | Maria et al, (2017). The role of Plant Growth Promoting Bacteria in improving nitrogen use efficiency for sustainable crop production: a focus on wheat, AIMS Microbiology, 3(3): 413-434. DOI: 10.3934/microbiol.2017.3.413. |
| [51] | S. K. Dubey et al, (2017). Effect of Azotobacter and Phosphobacteria on Yield of Wheat (Triticum aestivum), International journal of plant research, DOI: 10.5958/2229-4473.2017.00130.6. |
| [52] | Chennappa G., Sreenivasa M.Y., Nagaraja H. (2018). Azotobacter salinestris: A Novel Pesticide-Degrading and Prominent Biocontrol PGPR Bacteria. In: Panpatte D., Jhala Y., Shelat H., Vyas R. (eds) Microorganisms for Green Revolution. Microorganisms for Sustainability, vol 7. Springer, Singapore. |
| [53] | Spaepen S, Vanderleyden J, Remans R (2007). Indole 3 acetic acid in microbial and microorganism plant signaling. FEMS Microbiol Rev 31: 425–448. |
| [54] | Kadmiri, I.M., Chaouqui, L., Azaroual, S.E. et al. Arab J Sci Eng (2018). https://doi.org/10.1007/s13369-017-3042-9. |
| [55] | Li, H.Q. & Jiang, X.W. Russ J Plant Physiol (2017) 64: 235. https://doi.org/10.1134/S1021443717020078. |
| [56] | Ramazan et al., (2017). The Role of Soil Beneficial Bacteria in Wheat Production: A Review http://dx.doi.org/10.5772/67274. |
| [57] | Raouf Seyed Sharifi, Razieh Khalilzadeh & Jalal Jalilian (2016). Effects of biofertilizers and cycocel on some physiological and biochemical traits of wheat (Triticum aestivum L.) under salinity stress, Archives of Agronomy and Soil-Science, 63:3, 308-318, DOI: 10.1080/03650340.2016.1207242. |



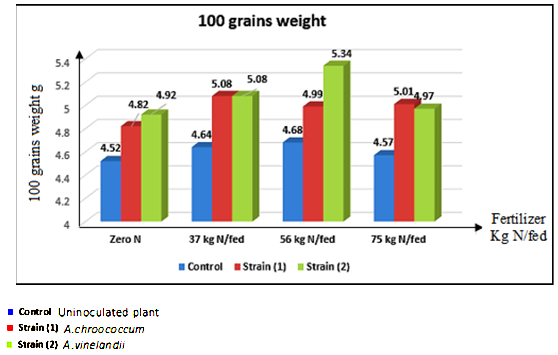
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML