-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2018; 8(2): 33-42
doi:10.5923/j.microbiology.20180802.02

Safe and Efficacious Anti-Cytomegalovirus Agents with Therapeutic Activity in vitro
Khalid A. El-Dougdoug1, Ahmed R. Sofy2, Adel A. Mousa2, Mahmoud R. Sofy2, Ahmed A. Hmed2, Ahmed A. Abbas2
1Virology Lab., Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
2Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
Correspondence to: Khalid A. El-Dougdoug, Virology Lab., Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The most widely used drugs for prophylaxis and treatment of CMV infections are chemical drugs. Unfortunately, these drugs have dose-limiting renal and bone marrow toxicities. Also, the long-term therapy with these drugs, necessary for management of infection, has been associated with the development of clinically resistant strains of the virus. Therefore, antiviral agents would need to develop for the treatment and suppression of CMV disease. Natural products of plant origin are proving to be a valuable source for anti-viral agents. So, the aim of the current study was to in vitro evaluation of Allium sativum and Nigella sativa methanolic extracts as anti-CMV agents. Murine Embryonic Fibroblasts (MEFs) were infected with CMV and propagative, plaque assay of CMV titer was 9.5x106 (PFU/ml) at four days post infection. Two methanolic plants extracts showed anti-cytomegalovirus (CMV) activity against CMV tissue culture which determined by cytopathic effects whereby, the number of staining survival cells were increased with the treatment by A. sativum or N. sativa methanolic extracts. While a mixture of both extracts showed a synergistic effect in controlling the virus greater than each extract in a solo state.
Keywords: CMV, ELISA, PCR, Virus, Garlic, Allium sativum, Nigella sativa, Antiviral, Plaque assay, Cytopathic effects
Cite this paper: Khalid A. El-Dougdoug, Ahmed R. Sofy, Adel A. Mousa, Mahmoud R. Sofy, Ahmed A. Hmed, Ahmed A. Abbas, Safe and Efficacious Anti-Cytomegalovirus Agents with Therapeutic Activity in vitro, Journal of Microbiology Research, Vol. 8 No. 2, 2018, pp. 33-42. doi: 10.5923/j.microbiology.20180802.02.
Article Outline
1. Introduction
- Cytomegalovirus is the most common cause of intrauterine infection, occurring in 0.2% to 2.2% of all live births and is a common cause of sensorineural hearing loss and mental retardation [1, 2]. Most healthy people who acquire CMV after birth experience few or no symptoms and no long-term sequelae. Some experience a mononucleosis-like syndrome with symptoms including malaise, persistent fever, myalgia, cervical lymphadenopathy, less commonly, pneumonia and hepatitis [3]. After the primary infection, defined as CMV infection in a previously seronegative person, the virus becomes dormant and exists in a latent state, from which it can be reactivated. This is designated as recurrent (secondary) infection [4]. Congenital infections are the result of transplacental transmission of CMV. Transmission to the fetus may occur because of primary or secondary maternal infection. The probability of intrauterine transmission following primary infection during pregnancy is 30% to 40% compared with only 1% following secondary infection [1, 3]. Ten to fifteen percent of congenitally infected infants will have symptoms at birth including intrauterine growth restriction, microcephaly, hepatosplenomegaly, petechiae, jaundice, chorioretinitis, thrombocytopenia, and anemia, and 20% to 30% of them will die, mostly of disseminated intravascular coagulation, hepatic dysfunction, or bacterial superinfection [3, 5]. Most of the congenitally infected infants (85–90%) have no signs or symptoms at birth, but 5% to 15% of them will develop sequelae such as sensorineural hearing loss, delay of psychomotor development, and visual impairment [6].The most widely used drugs for prophylaxis and treatment of CMV infections are foscarnet, cidofovir, and ganciclovir. Unfortunately, these drugs have dose-limiting renal and bone marrow toxicities. Also, the long-term therapy with these drugs, necessary for management of infection, has been associated with the development of clinically resistant strains of the virus [7]. Therefore, antiviral agents would need to develop for the treatment and suppression of CMV disease. Natural products of plant origin are proving to be a valuable source for anti-viral agents such as Nigella sativa and garlic [8, 9]. Therefore, the current study was aimed to in vitro evaluation of A. sativum and N. sativa methanolic extracts as anti-CMV agents.
2. Materials and Methods
2.1. Plant Materials
- Seeds of Nigella sativa and bulbs of garlic (Allium sativum) were obtained and identified in Horticulture department, Fac. of Agric. Ain Shams Univ. The collected plants parts were cleaned, washed and shade dried, exposure to sunlight and avoided to prevent the loss of active components.
2.2. Preparation of Methanol Extract
- The dried materials from each plant weighing 100 (g) were ground to fine powders by the electric grinder. A measured quantity of dried powder was then macerated in 1000 ml absolute methanol in properly covered and labeled in a conical flask at room temperature for 72 h. Each extract was filtered with (Whatman No.1) filter paper. The filtrate was evaporated to dryness at 40°C in a vacuum using a rotary evaporator until dry methanol extract was obtained. The weight of the dried extract was 5.6 (g), and 6.3 (g) for N. sativa and garlic, respectively was obtained. The extracts were stored in airtight containers at 4°C for further use [10].
2.3. Cell Culture Type
- Due to their high productivity, Murine Embryonic Fibroblast (MEFs) are the cells of choice for virus stock production. Fibroblast cell lines such as NIH 3T3 or BALB/c 3T3 also used for propagation of CMV; however, these cell lines tend to grow very fast, and virus plaques are more difficult to visualize by microscopic inspection due to the rapid growth of surrounding cells according to [11, 12].
2.4. Preparation and Propagation of CMV Stocks
- Virus stock preparation and titration. Cell-free virus stocks (CMV) were tittered by serial 10-fold dilution in tissue culture plates containing human fibroblast monolayers overlaid with agarose. After incubation for 7 to 10 days, the cultures were fixed, and plaques were enumerated. PFU per milliliter were determined. The virus was quantitated by estimating the number of plaque-forming cells (PFC) present in an infected culture [13]. When viral cytopathic effects (CPE) involved approximately 70 to 80% of the monolayer, the cells were trypsinized and resuspended in minimal essential medium (MEM) with 8 to 10% fetal bovine serum (FBS), and an aliquot was counted in a hemacytometer. The number of PFC was then determined by multiplying the number of cells in the suspension by the percentage estimated to be infected, as evidenced by CPE. Cell concentration was adjusted to 400 PFC/ml in 2% MEM to provide an inoculum dose of 60 to 80 PFC/0.2 ml. The plates were inoculated on the same day, and the infected cell suspension was centrifuged at low speed (250 × g for 10 min). The cell pellet was resuspended in cryoprotective medium (20% FBS–10% dimethyl sulfoxide–MEM). The cell suspensions were frozen slowly at a titer of 4 × 105 PFC/ml and stored at −80°C until use when they were thawed quickly and diluted in MEM with 2% FBS to obtain a concentration of 400 PFC/ml [14].
2.5. Antiviral Activity of Herbal Extracts
2.5.1. Plaque Reduction Assay Using Tissue Culture Method [Virucidal Activity]
- Complete medium stainless-steel wire mesh (0.45-mm grid size) Additional reagents and equipment for parenteral injection and determining viral titer in tissues. Infection of newborn mice: Inject newborn BALB/c mice intraperitoneally with 10 to 100 pfu-purified CMV. Intraperitoneal injection of newborn mice should not perform directly through the abdominal wall. To prevent the return of injected fluid, the needle should insert in the thoracic region and passed under the skin before it enters the peritoneal cavity. Newborn mice get sick after infection with as little as 100 pfu, Infection of newborns with 1000 pfu results in severe disease (ranting, hair loss, general failure to thrive) and very high mortality rates. Euthanize animals on days 7, 14, and 21 postinfection and remove organs (e.g., kidney, and lunge). Freeze whole organs individually at -70°C in plastic tubes until titration can be done. Thaw mouse organs and homogenize them by passing them through a 0.45-mm stainless steel wire mesh. Use the plunger of a syringe (or a similar sterile device) to squeeze the organ tissue through the wire mesh, take one layer (cell beside cell) from mice kidney, and lunge cells that infected by CMV to perform tissue culture as positive control, and other infected layers with treatment using plants methanolic extracts for determination of antiviral activity [The following final extract concentrations for both extracts were used in the assay, 0, 5, 15, 25, 35, 45, 55, 65, 75, 85 and 95μL]. As well as, take one layer (cell beside cell) from mice kidney, and lunge cells that not infected by CMV to perform tissue culture as a negative control. Collecting the homogenized material in a small petri dish. Rinse the mesh with 4 ml complete medium to recover residual material; this will result in 5 ml total volume. Immediately, determine CMV titer in organ homogenate dilutions using the plaque forming cell assay. Organ homogenates can be toxic for cultured cells especially when used at low dilutions. This most frequently observed for kidney and lunge. Therefore, it might be necessary to start titration at a higher dilution (e.g., 1/2000) for these organs [15].
2.5.2. Determination of the Anti-Infectivity Effect of Extracts [Using Cell Viable Method]
- 0.1 (ml) of CMV (containing approximately 9.5 x 106 plaque forming units/ml) was mixed with 0.1 (ml) of experimented extract (selected dilution). The mixtures were incubated at room temperature for one hour in sterile screw-capped vials. Non-treated virus infected control set was done by mixing 0.1 ml of (103) of virus suspension in MEM, with 0.1 (ml) of MEM medium [virus control]. Then 12- well plates seeded with Murine Embryonic Fibroblast cells (MEFs) were washed with wash solution (total washes 2) then added 1 ml wash solution and incubated at room-temperature for 5-10 minutes. 0.2 ml of either test or a control vial was inoculated. The plates were incubated at 37C (to allow virus adsorption) for one hour. The daily observation was carried out for detection of virus effect. Cells were fixed by flooding with 10 % formalin solution for one hour at room-temperature. This treatment inactivated the virus. Then cell monolayers were washed under tap water to remove the remaining overlay and stained with Haematoxylin and Eosin staining solution for 15 minutes; the excess dye was washed off [15].
2.6. Histological Examination of the Liver and the Lungs Tissues
- Liver and lungs were isolated on day 16 after infection and fixed in 10% formalin solution. The specimens were dehydrated and embedded in paraffin. 4 μm thick sections were cut and stained with hematoxylin and eosin and then examined by light microscopy [16].
2.7. A Phytochemical Study [Gas Chromatography-Mass Spectrometry (GC-MS) Analysis]
- The chemical composition of both extracts samples was performed using Trace GC1310-ISQ mass spectrometer (Thermo Scientific, Austin, TX, USA) with a direct capillary column TG5MS (30 m x 0.25 mm x 0.25 µm film thickness). The column oven temperature was initially held at 50°C and then increased by 5°C /min to 200°C hold for 2 min increased to the final temperature of 280°C by 30°C /min and hold for 2 min. The injector and MS transfer line temperatures were kept at 270, 250°C respectively; Helium was used as a carrier gas at a constant flow rate of 1 ml/min. The solvent delay was 3 min, and diluted samples of 1 µl were injected automatically using autosampler AS1300 coupled with GC in the split mode. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 45–500 in full scan mode. The ion source temperature was set at 200°C. The components were identified by comparison of their retention times and mass spectra with those of WILEY 09 and NIST 11 mass spectral database.
3. Results
3.1. Cytopathic Effect of Cytomegalovirus
- Cytomegalovirus produced a cytopathic effect in MEF cells within four days post infection; the most common effects begin with cytoplasmic granulation after which, the cells become rounded, longed, multinucleated giant and swelling cells. Their macrocytes become refractive in appearance and undergo lytic degeneration (Fig. 1).
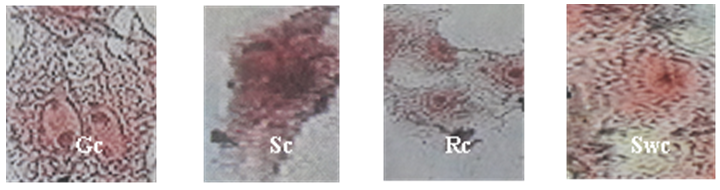 | Figure (1). Photogram of cell lines, cytopathic effect of CMV on cell morphology. Gc= Giant cell, Sc= Spinal cell, Rc= Round cell, and Swc= Swelling cell |
3.2. Cytomegalovirus Plaque Inhibition Assay
- Cytomegalovirus titer calculated as plaque forming unit (PFU/ml) on the infected monolayer cell line. CMV titer plaque inhibition assay of methanolic N. sativa and A. sativum extracts were 55 µL and 45 µL, respectively (CMV titer was 9.5x106 PFU/ml) at four days post-infection in murine embryonic fibroblasts (MEFs) monolayer cells line under agarose over layer as shown in figure (5). While CMV titer plaque inhibition assay of a mixture of both methanolic extracts was 25 µL.
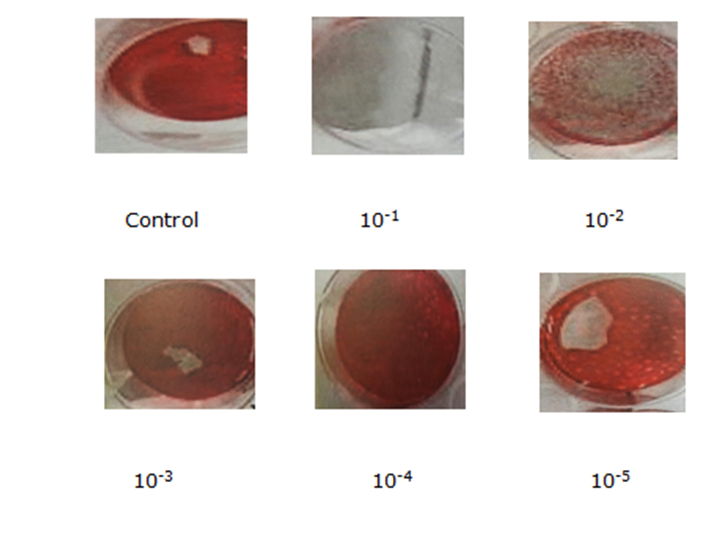 | Figure (5). MEF cell line inoculated with isolated CMV showing increasing plaques number with virus concentration |
3.3. Effect of CMV on Cell Viability
- Hematoxylin was recommended in cytopathology staining (as a nuclear counter stain). The obtained results showed that nuclear chromatin appeared blue and visible. Cytoplasm displayed various shades to pink-orange (depended upon counter stain). It was applied on cell suspension and cell line prepared from MEF. All cell line and cell suspension showed be considered potentially virus infectious and showed CPE of CMV on the cell line (giant cells, rounded, and swelling). The investigation revealed several cytoplasm displayed various shades to pink-orange, and nuclear chromatin showed blue in several MEF cells line. Therefore, some cell culture and tissue should be considered potentially infections were stained with hematoxylin and eosin. The numbers of non- stained survival cells were 80 cells, while the stained CMV infected dead MEF cells were 160. On the other hand, the numbers of non- stained MEF cells treated with garlic increased were 123 cells, while the stained CMV infected dead MEF cells line decreased to 117 cells.As well as, the numbers of non- stained MEF cells treated by N. sativa increased were 101 cells, while, the stained CMV infected dead MEF cells line decreased to 139 cells lines. Whereas, the numbers of non- stained MEF cells treated by both garlic, and N. sativa mixture extract increased to 192 cells, while the stained CMV infected dead MEF cells decreased to 48 cells as shown in the table (1).
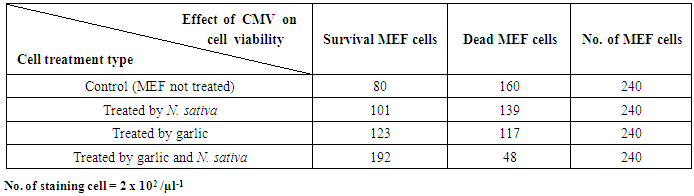 | Table (1). Effect of treatment CMV with plant extracts on viability cell suspension as no. of staining survival cells |
3.4. Identification of Phytochemical Compounds of A. sativum Extract Using GC-Mass
- The GC-MS results showed 33 compounds were detected. The most compounds with high areas percentages were 1-(2,4-Dimethoxy-phenyl)-pentan-3-one (2.11%), 1-Hexadecanol, 2-methyl-2-Methylhexadecan-1-ol (1.84%), Dotriacontane (2.79%), 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester (3.14%), 9,12-Octadecadienoic acid (Z,Z)-, 2,3-bis[(trimethylsilyl)oxy] propyl ester (32.33%), 1-Monolinoleoylglycerol trimethylsilyl ether (24.90%), 9,12,15-Octadecatrienoic acid, and 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl) oxy] methyl] ethyl ester (24.90%). The antiviral activity was attributed to some of these compounds (Table 2 & Fig. 7).
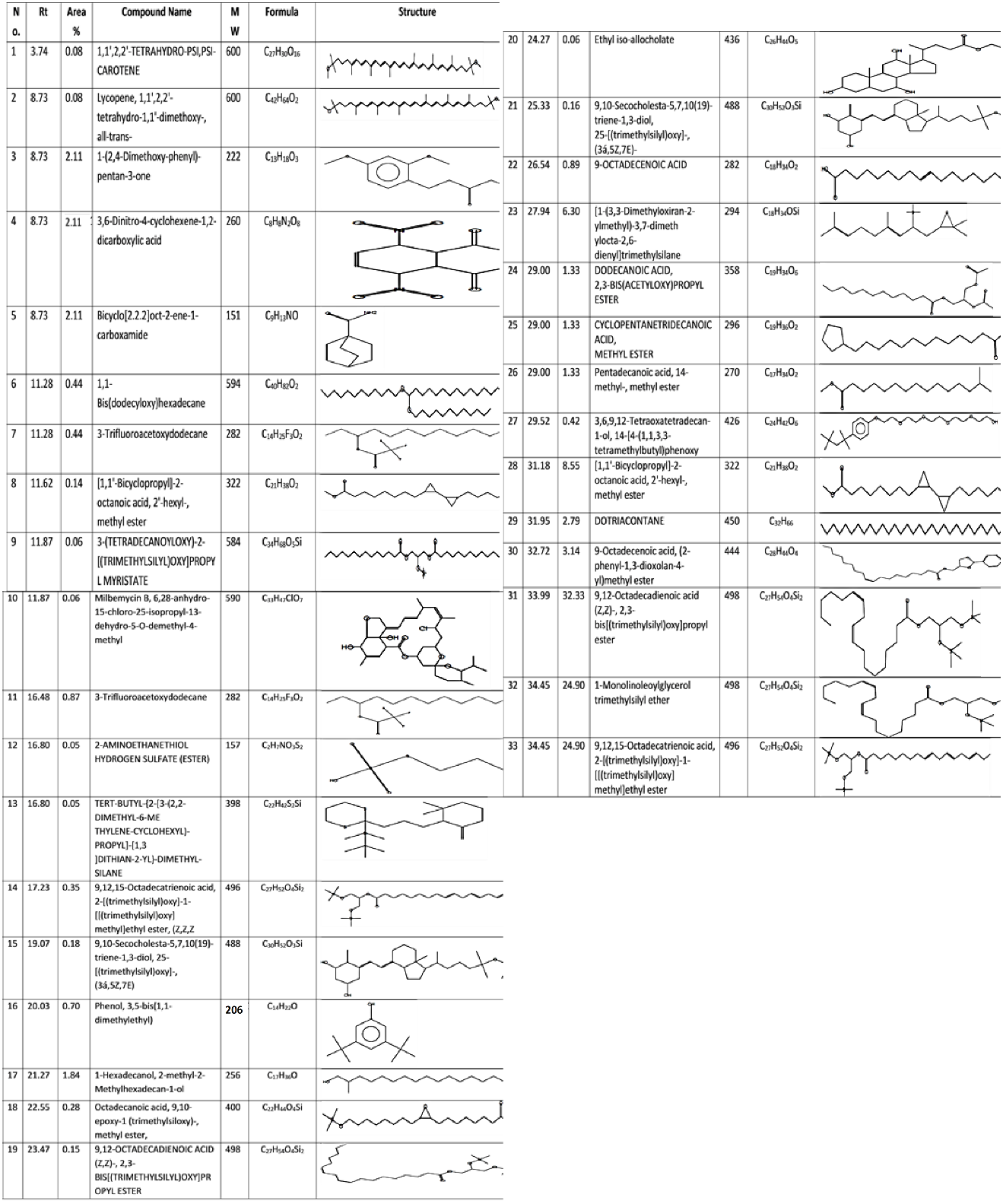 | Table (2). Major phytochemical compounds identified in methanolic extract of A. sativum by GC-Mass |
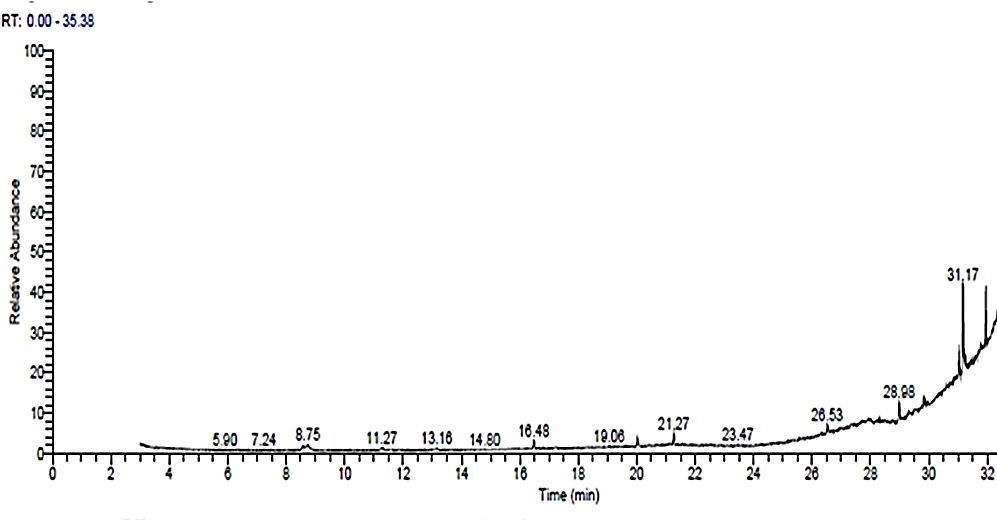 | Figure (7). GC-MS Chromatogram of methanol A. sativum extract |
3.5. Identification of Phytochemical Compounds of N. sativa Methanolic Extract Using GC-Mass
- Gas chromatography and mass spectroscopy analysis of N. sativa showed 62 compounds were detected the most compounds with high areas percentages were (Z,E)-à-Farnesene (19.73%), (2E,6E,9E)-2,6,10-TRIMETHYL-2,6,9,11-Dodecatetraenal (19.73%), p-Benzoquinone, 2-tert-butyl (11.22%), p-Mentha-3,6-diene-2,5-dione (11.22%), 1,3,3 Trimethylbicyclo [2.2.1] hept-2-yl pentanoate (2.36%), p-Cymene (16.94%), o-Cymene (16.94%), p-Mentha-1,8-diene, (D-Limonene) (7.68%), and 3-Thujene (3.74%). The antiviral activity was attributed to some of these compounds (Table 3 & Fig. 8).
 | Table (3). Major phytochemical compounds identified in methanolic extract of N. sativa by GC-Mass |
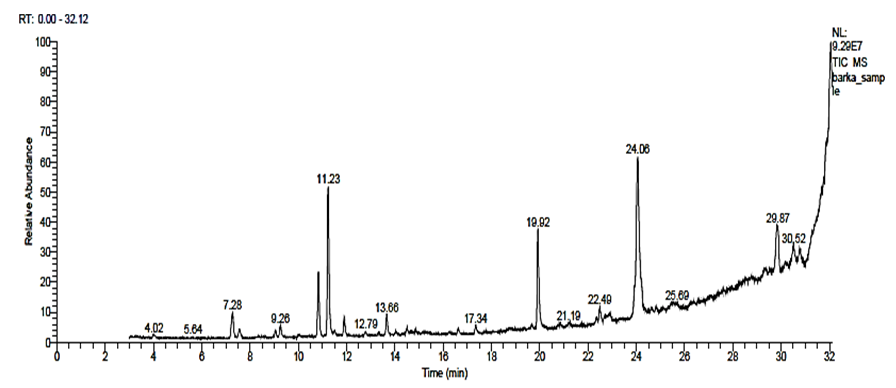 | Figure (8). GC-MS Chromatogram of N. sativa methanol extract |
4. Discussion
- Cytomegalovirus is the most common cause of intrauterine infection and is a common cause of sensorineural hearing loss and mental retardation [1, 2]. Although specific antiviral drugs, such as ganciclovir and foscarnet, have been available for several years for treatment of life-threatening or sight-threatening CMV disease in immunocompromised patients, their use for treatment of congenital CMV infection remains undefined due to a paucity of data. In principle, two levels of treatment could be considered, prenatal (during fetal life) and postnatal (based on the severity of clinical symptoms). Foscarnet is a competitor of pyrophosphate, while ganciclovir acts as a competitor of guanosine during viral DNA synthesis [17, 18]. However, the degree of toxicity of the two drugs must be carefully considered, with special regard to the renal toxicity of foscarnet and the hematologic toxicity of ganciclovir [17, 18]. Many natural agents including plant extracts showed antiviral activity. In the current study garlic and N. sativa methanolic extracts were examined for antiviral activity against CMV. CMV is characterized by a long-life cycle (4–5d), and a peculiar cytopathic effect, which is observable on many different CMV infect able cell types [19, 20], this characteristic cytopathic effect shaped the name of the virus. The data reported in this study provide a molecular explanation for the cytopathic effect of CMV, which can have treated with N. sativa and garlic extracts.From the obtained results in our study CMV titer plaque inhibition assay of methanolic N. sativa extract concentration was 55 µL; CMV titer Plaque inhibition assay of methanolic garlic extract was 45 µL; while, CMV titer Plaque inhibition assay of methanolic N. sativa and garlic extracts were 25 µL against CMV with titer concentration 9.5x106 (PFU/ml) at 4 days post-infection in Murine embryonic fibroblasts (MEFs). These findings demonstrate the antiviral activity of N. sativa and garlic extracts against CMV. These results stipulate significant capacity and future scope for the use of this bioactive compound for new anti-viral drug development. Active compounds of N. sativa and methanolic garlic extracts showed different compounds which may be attributed to antiviral properties of both extracts. These compounds from two plants are considered to be one of the natural bases for the production of bioactive compounds. Many previous studies are supporting these findings which fight against pathological conditions, and many of them are in the same line of herbal medicines [21]. The usage of herbal medicine has amplified dramatically for various diseases amongst general people over last few years not only because of their easy accessibility without prescription, low cost, and appointment to the health care specialists and more with the belief that, but natural remedies also have less lethal effects as compared to synthetic medicines [22]. GC-MS of garlic methanol extract recorded the highest level of allicin giving area (24.90%), significant variations were found between the allicin content of the commercially produced garlic products because they were prepared under different manufacturing conditions and with different chemical specifications and good yield of other compounds. Our findings are in agreement with previous results by Tsai et al. [23], which indicated that, 9, 12, 15-Octadecatrienoic acid and 2- [(trimethylsilyl)oxy]-1- [[(trimethylsilyl)oxy] methyl] ethyl ester were two compounds identified from garlic methanol extract and showed an antiviral activity against influenza virus and CMV. Also, garlic extracts have shown a significant effect on the growth of CMV on tissue culture. These findings are in consistency with earlier reports that stated extracts of garlic could inhibit the growth of virus on tissue culture, and the effectiveness of this inhibition is related to the solvent used in the extraction [24]. Many previous studies reported that the antiviral activity of garlic is related to allicin, which is the main biologically active component of garlic extract inhibiting viral growth such as common cold virus [25], CMV [26-28], Rhinovirus and Herpes simplex virus type 1 [23]. Similarly, ajoene (allicin derivative) has also shown strong inhibitory effect against several viral species including CMV [29] and subsequent other viruses [30]. Furthermore, it was found that, some other compounds from garlic extract have antiviral activity against CMV such as dotriacontane [31], [1-(3,3-Dimethyloxiran-2-ylmethyl)-3,7-dimethylocta-2,6-dienyl] trimethylsilane [32], [1,1'-Bicyclopropyl]-2-octanoic acid, 2'-hexyl-, methyl ester [33] and 9,12-Octadecadienoic acid (Z,Z)-, 2,3-bis[(trimethylsilyl)oxy] propyl ester [34]. Also, this may be attributable to the high concentration of sesquiterpene as well as phenolic compounds present in the methanolic fraction [35]. A qualitative investigation of N. sativa has revealed the presence of sterols, triterpenes, tannins, flavanoids, cardiac glycosides, alkaloids, saponins, volatile oils, volatile bases, glucosinolates, and anthraquinones [36]. Qualitative evaluation of the black seed oil via capillary GC-MS technique has enabled the identification of 67 compounds, when classified into various functional groups corresponding with the following data: monoterpenes (~46%); carbonyl compounds (~25%); phenols (~1.7%); alcohols (~0.9%) and esters (~16%) [37]. These findings are in harmony with Abdel-Shafi [38], who suggested that the antiviral activity may be attributed to phenolic compounds in plant extract. In respective to the mechanism of inactivation, Isaacs et al. [39] concluded that the activity of the plant extracts was due to the interaction of the polyphenol compounds with the viral capsid of the non-enveloped viruses. For the enveloped virus such as HSV, inactivation was due to the polyphenol directly binding to fusion proteins or binding to the viral envelope glycoprotein. As well as, the presence of saponins, alkaloids, glycosides, tannins, carbohydrates, flavonoids, resins, acidic compounds and proteins. Plant-derived flavonoids have been reported to inhibit critical steps in the life cycle of viruses including HIV infectivity such as viral entry [40], reverse transcriptase [41], integrase [42], viral transcriptional activities [43] and protease inhibitory activities [44] and other activities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML