-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2018; 8(2): 23-32
doi:10.5923/j.microbiology.20180802.01

Characterization of Bacillus subtilis Available as Probiotics
Ana Carolina Ritter, Ana Paula Folmer Correa, Flávio Fonseca Veras, Adriano Brandelli
Laboratório de Bioquímica e Microbiologia Aplicada, Instituto de Ciência e Tecnologia de Alimentos, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Correspondence to: Ana Carolina Ritter, Laboratório de Bioquímica e Microbiologia Aplicada, Instituto de Ciência e Tecnologia de Alimentos, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Probiotic microorganisms are generally considered to promote the balance of intestinal microbiota and generate health benefits to the host. However, selection of probiotics for incorporation into specific diets requires a scrutiny in the form of both in vitro and in vivo tests. In this work, in vitro probiotic properties of Bacillus subtilis strains available in probiotic formulations were investigated. The isolates CHR01 and FTC01 showed broad antimicrobial spectra, inhibiting Gram-positive bacteria and Aspergillus species, but only Aeromonas hydrophila was inhibited among Gram-negative bacteria. Highest antioxidant activity was measured for isolates KM01 and FTC01. A marked difference in adhesion to hydrocarbons and auto-aggregation properties, ranging from 2.2 to 56.4% and 4.4 to 52.9%, respectively, was observed among the tested bacteria. The Bacillus isolates were mostly tolerant to bile salts and acid pH, although the strains FPR02 and CHR01 were sensitive to ox bile. Among tested cultures, FTC01 and CP01 showed more than 90% survival at pH 2. None Bacillus tested showed positive hemolytic reaction. Four strains were evaluated for surfactin production and higher production was observed by Bacillus FPR01, independently of the substrate.
Keywords: Bacillus, Probiotic, Surfactin, Beneficial microorganism
Cite this paper: Ana Carolina Ritter, Ana Paula Folmer Correa, Flávio Fonseca Veras, Adriano Brandelli, Characterization of Bacillus subtilis Available as Probiotics, Journal of Microbiology Research, Vol. 8 No. 2, 2018, pp. 23-32. doi: 10.5923/j.microbiology.20180802.01.
Article Outline
1. Introduction
- Probiotic microorganisms are generally considered to promote the balance of intestinal microbiota and generate health benefits to the host [16]. The modulation of the immune system, decrease of serum cholesterol levels, prevention of intestinal disorders, such as diarrhea or lactose intolerance, and antibody-associated diarrhea are some of the benefits presented by these bacteria and yeasts [23].Although different microorganisms are claimed today as probiotics, most descriptions are associated with the genera Lactobacillus and Bifidobacterium [23]. The Bacillus species constitutes an interesting group of probiotic bacteria that received limited attention. These microorganisms are continuously studied and have demonstrated high probiotic potential, since Bacillus can survive in foods that require harsh processing conditions, such as high temperature and pressure, survive better during the gastrointestinal transit, have a long shelf life and remain viable throughout their lifetime, both at room temperature and under refrigeration conditions [12, 41]. Due to its better survivability, the effective dose required for Bacillus as probiotic supplements is usually lower than that required for lactic acid bacteria [12, 15]. Despite the benefits associated with Bacillus, safety evaluation of potential probiotic strains seems mandatory. Foodborne illnesses and production of toxins have been associated with species like Bacillus cereus and Bacillus anthracis, but in some cases with species often regarded as safe, such as Bacillus subtilis [15].Bacillus could play a significant role in the gut because of their high metabolic activity, which is largely determined by their ability to synthesize antimicrobials. There were identified more than 700 antibiotics from Bacillus spp. [2]. The most studied species is B. subtilis, which devotes 4-5% of its genome to antibiotic synthesis and produce diverse antibiotics [43]. Antimicrobial compounds produced by Bacillus have a variable spectrum of antimicrobial activity. Some strains can be effective against bacteria of related species, while other strains can produce a wide spectrum of antimicrobial activity, including antifungal and antiprotozoal substances [1, 41]. A vast array of structurally unrelated antimicrobial compounds, which include lipopeptides like surfactin and iturin, are produced by some Bacillus isolates [43]. These lipopeptides are recognized by varied biological activities, including antifungal, antiviral, antitumor, blood anticoagulant and fibrinolytic activities, as well as ability to stimulate responses related to immune system in host tissues [34, 42].Despite some Bacillus strains are commercially available as probiotics, detailed studies about the properties of these strains are scarce [14]. These bacteria can exert their beneficial effects either by competing with undesirable microbiota, by favouring beneficial microbiota, or by producing metabolites that are useful to maintain healthy microbiota. In general, studies on commercial Bacillus probiotics intended for animal nutrition have been developed by inclusion in diets and compared with a standard diet in vivo [17, 24]. However, comparative studies among commercial cultures either in vivo or in vitro are rare. Therefore, the aim of this study was to evaluate the in vitro probiotic characteristics of different B. subtilis obtained from commercial formulations, in addition to quantify the production of surfactin by some isolated Bacillus.
2. Materials and Methods
2.1. Bacterial Strains
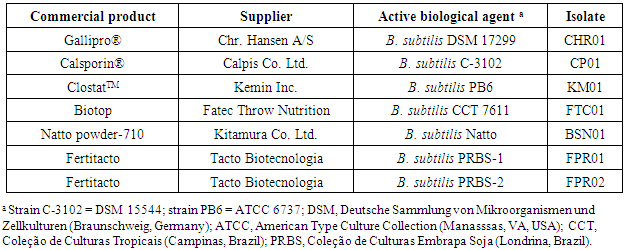
- Bacteria used in this study were isolated from commercial brands based on B. subtilis as the biological agent (Table 1). After serial dilution in peptone water and spread plating onto BHI broth, followed by incubation under aerobic conditions at 37°C for 24 h. Discrete bacterial colonies were picked from each product and identified as follows: CHR01 (Gallipro), FTC01 (Biotop), CP01 (Calsporin), KM01 (Clostat) and BSN01 (natto), and FPR01 and FPR02 (Fertitacto). B. subtilis DSM 4451 (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) was used as a reference strain. Characterization of the isolates included morphological, cultural, physiological and biochemical features. All tests were carried out following standard procedures [8, 27].
|
2.2. Determination of Antibacterial Activity
- Antibacterial activity was determined according to Motta and Brandelli [30], with some modifications. The test-cultures were Bacillus cereus ATCC 9634, Bacillus subtilis ATCC 9372, Clostridium perfringens ATCC 3624, Staphylococcus aureus ATCC 1901, Salmonella enterica subsp. enterica ATCC 13076, Escherichia coli ATCC 8739, Aeromonas hydrophila ATCC 7966 and Pseudomonas aeruginosa 4B. The last two strains were obtained from the culture collection of Laboratório de Microbiologia de Alimentos (Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil). Test microorganisms, at a concentration of 108 CFU/ml in saline solution (0.85% NaCl, w/v), were inoculated with a swab onto BHI agar plates. Aliquots of 15 µl of crude supernatant of Bacillus cultures were spotted on the freshly prepared lawn of test-cultures, and plates were incubated aerobically at the optimal temperature for each test microorganism. The plates inoculated with C. perfringens were incubated in an anaerobic jar supplied with a disposable anaerobic atmosphere generator (Anaerobac, Probac, São Paulo, Brazil) at 37°C. Subsequently, zones of growth inhibition (represented by clear haloes) were measured and the antagonistic activity was presented as inhibition diameters (mm).
2.3. Determination of Antifungal Activity
- To determine the antifungal activity of the Bacillus cultures, the filamentous fungi Aspergillus niger, Aspergillus flavus, Aspergillus carbonarius, and Aspergillus parasiticus, Penicillium citrinum, Penicillium chrysogenum, Penicillium herquei and Fusarium oxysporum were utilized as test cultures [47]. All fungi are kept in the culture collection of the Laboratório de Toxicologia de Alimentos (Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil). The fungi were cultivated in Potato Dextrose Agar (PDA) plates for 5 days at 30°C. Conidia suspensions of the filamentous fungi were prepared, and then added to PDA at 50°C in a sufficient volume to provide a final concentration of 106 conidia/ml. The medium was poured onto plates and, after solidification, 15 µl of the crude supernatant of Bacillus cultures were spotted. The plates were incubated at 30°C for 48 h and subsequently observed for inhibitory activity against the fungal indicator.
2.4. Activity Determination of Reducing Power
- Reducing power of the Bacillus cultures was measured as previously described by Zhu et al. [50]. Samples of crude supernatants (1 ml) were mixed with 2.5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide (10 mg/ml), and the mixture was incubated at 50°C for 20 min. Then, 2.5 ml trichloroacetic acid (10%, w/v) was added and the mixture was centrifuged (3000 × g for 10 min). The supernatant (1 ml) was mixed with 2.5 ml distilled water and 0.2 ml ferric chloride (1 mg/ml), and the absorbance was measured at 700 nm. Higher absorbance of the reaction mixture indicated greater reducing power. Butylated hydroxytoluene (BHT) was used as a positive control.
2.5. DPPH Radical-Scavenging Assay
- This method, performed as described by Brand-Williams et al. [3], is based on the capture of the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical by antioxidants, producing a decrease in absorbance at 517 nm. The DPPH was used at a concentration of 60 µM, dissolved in methyl alcohol. The solution was homogenized and transferred to a dark glass bottle. The prepared solution was used only on the day of analysis. In the dark, aliquots of 0.1 ml sample (crude supernatants) were transferred to test tubes with 3.9 ml radical DPPH solution and homogenized by shaking. After 45 min, the scavenging activity was measured spectrophotometrically by the decrease in absorbance at 517 nm. Likewise, these same proportions (0.1 ml distilled water and 3.9 ml DPPH radical) were used as a control. Methyl alcohol was used as a blank. The standard curve was performed using DPPH concentrations from 0 to 60 µM. The results were expressed as:
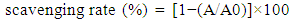 | (1) |
2.6. Bacterial Adhesion to Hydrocarbons (BATH) and Auto-Aggregation Tests
- BATH and auto-aggregation ability of the cultures were assessed according to the procedure described by Canzi et al. [4]. BATH test was conducted using hydrocarbon xylene. A 16 ± 2 h grown culture (1 ml, approximately 107 CFU/ml) was taken and centrifuged at 8,000 x g for 15 min at 4°C. The collected pellet was washed with phosphate-buffered saline (PBS: 140 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4, pH 7.2) and suspended in the same buffer to an absorbance (600 nm) of 0.5. An equal volume of xylene was added and the two phase system was thoroughly mixed by vortexing for 3 min. The aqueous phase was removed after 1 h incubation at 27 ± 2°C and the absorbance was measured at 600 nm. Adhesion percentage was calculated using the formula:
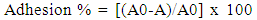 | (2) |
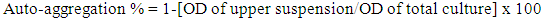 | (3) |
2.7. Bile Tolerance Test
- Growth in the presence of 0.3% (w/v) ox bile was analyzed as described by Gilliland et al. [19]. Test cultures were grown overnight (16 ± 2 h at 37°C), centrifuged at 8,000 x g for 15 min at 4°C and the pellet collected was suspended in same volume of saline (0.85% NaCl). Fresh LB broth (5 ml), without ox bile (for control), and LB broth (5 ml) containing 0.3% (w/v) ox bile was inoculated with 250 μl (5%) of cell suspension. The growth was monitored hourly by measuring the OD at 650 nm. Time lag to reach log phase at various bile concentrations was determined to ascertain bile tolerance or sensitivity of the cultures [6]. The cultures were categorized into four groups according to the observed delay of growth (d) in the presence of ox bile: resistant strains (d≤15 min), tolerant strains (15<d≤40 min), weakly tolerant strains (40<d<60 min), and sensitive strains (d≥60 min).
2.8. Acid Tolerance Test
- Tolerance to low pH was tested for the bacterial cultures as described by Conway et al. [10]. For this purpose, active cultures (incubated for 16 ± 2 h, approximately 107 CFU/ml ) were used. Cells were harvested by centrifugation for 15 min at 8,000 x g and 4°C. Pellets were washed once in phosphate-saline buffer (PBS at pH 7.2), suspended in PBS (pH 2.0 or 3.0) and incubated at 37°C. Surviving microorganisms were enumerated at 0 and 2 h by plating in LB agar and expressed as colony-forming units (CFU) per milliliter. The survival rate was calculated using the formula [18]:
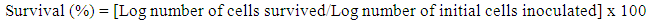 | (4) |
2.9. Exopolysaccharide Production
- Exopolysaccharide (EPS) production was examined as reported previously [29]. Briefly, 16 ± 2 h grown Bacillus cultures (approximately 107 CFU/ml) were streaked on the surface of plates containing ruthenium red milk (100 g/l skim milk powder, 10 g/l sucrose, 0.08 g/l ruthenium red, and 150 g/l agar). After incubation at 37°C for 24 h, ropy white colonies were scored as EPS-producing strains.
2.10. Phytase Activity
- Phytate degradation plate assay was performed as described by Raghavendra and Halami [37], using a modified LB medium containing 6.25 g/L sodium phytate. The harvested cell suspension (3 μL of 107-108 CFU/ml) was spotted onto the surface of modified LB agar and incubated for 16 ± 2 h at 37°C. After incubation, colonies were washed from the agar surface using double-distilled water and flooded with 20 g/l cobalt chloride solution. After 5 min of incubation at 27 ± 2°C, the cobalt chloride solution was replaced with a freshly prepared solution containing equal volumes of 62.5 g/l ammonium molybdate and 4.2 g/l ammonium metavanadate aqueous solutions. After 5 min of incubation, the ammonium molybdate/ammonium vanadate solution was removed and the plates were examined for zones of phytate hydrolysis.
2.11. Cellulolytic Activity
- The evaluation of cellulolytic activity was performed using a modified Luria-Bertani medium (LB) containing 5 g/l carboxymethylcellulose (CMC) [5]. After the growth of the microorganisms by 24 h, the revelation of the activity was performed by adding to each plate 10 ml of 2.5 g/l Congo Red solution prepared in Tris-HCl buffer (0.1 M, pH 8.0) for 30 min. After this period, the plates were washed with 5 ml of NaCl (0.5 M) for 5 min. Thereafter the plates were examined for clear zones around the colonies.
2.12. Haemolytic Activity
- The Bacillus strains were plated on blood agar plates containing 5% (v/v) sheep blood and incubated for 24 h at 30°C [28]. Hemolytic reaction was classified by observing a partial hydrolysis zones in green tones around the colonies (α-hemolysis), clear zone around the bacterial growth (β-hemolysis), and no reaction (γ-hemolysis).
2.13. PCR Identification of Surfactin and Iturin Genes (sfp and ituD)
- DNA was extracted from overnight cultures using the Promega Wizard SV Genomic DNA kit (Promega, Madison, WI, USA).The specific primers used for PCR amplification of the sfp and ituD genes were developed by Hsieh et al. [20]. The primers sfp-f (5’-ATGAAGATTTACGGAATTTA-3’) and sfp-r (5’-TTATAAAAGCTCTTCGTACG-3’) were used to amplify a 675-bp sfp fragment corresponding to the B. subtilis surfactin gene cluster, whereas the primers ituD-f (5′-ATGAACAATCTTGCCTTTTTA-3′) and ituD-r (5′-TTATTTTAAAATCC GCAATT-3′) were employed for the 1,203-bp ituD fragment, corresponding to the Bacillus amyloliquefaciens iturin A. Each 50 μl of PCR mix contained 5 μl of Taq buffer 10x, 3 μl of 25 mM MgCl2, 0.4 μl of 25 mM dNTPs, 0.5 μl of Taq polymerase (5 U/ml; Invitrogen), 1.25 μl of 20 μM primer, 50 ng of genomic DNA and 36.1 μl of ultrapure H2O. PCR was performed using a Mastercycler Personal thermocycler (Eppendorf AG, Hamburg, Germany) under the following conditions: denaturation for 1 min at 94°C, annealing for 1 min at 50°C, and elongation for 1.5 min at 72°C for a total of 30 cycles for iturin A, and denaturation for 1 min at 94°C, annealing for 30 s at 46°C, and elongation for 1 min at 72°C for a total of 25 cycles for surfactin.
2.14. Surfactin Production
- The ability of test bacteria to produce surfactin isomers was assessed in different growth substrates. Bacterial cells were grown in 250 ml Erlenmeyer flasks containing 50 ml of Tryptic soy broth (TSB), 10 g/l soybeans, extruded soy or soybean meal, at 37°C and 125 rpm for 48 h. After growth, the supernatant and cell pellets were separated by centrifugation at 10,000 x g for 15 min at 4°C. The obtained material was subjected to extraction of surfactin. The supernatant was added to 12.5 ml n-butanol, vortexed for 1 min and incubated overnight at room temperature in order to extract extracellular lipopeptides. The organic phase was taken and the solvent removed by evaporation at 30°C and then the material obtained was dissolved in 2 ml methanol before quantification analysis [49].Quantitative analysis was performed by high performance liquid chromatography, using an E2695 HPLC (Waters, Milford, MA, USA) equipped with a photodiode array detector (PAD-2998). A 40 µl aliquot of each sample was injected into C18 column (XBridgeTM Shield RP 18; 5 μm; 4.6 x 150 mm, Waters) and column oven temperature was set to 20°C. The surfactin analysis was performed using a mobile phase consisting of methanol:trifluoroacetic acid (0.1%) (90:10, v/v). The method was developed in isocratic system at 0.3 ml/min and three surfactin isoforms (C-13, C-14 and C-15) were monitored at 210 nm [26].Appropriate dilutions of surfactin (Sigma, St. Louis, MO, USA) were made with methanol to obtain standard solutions (20, 40, 60, 80, 100, 120 and 140 mg/l) in order to develop a calibration curve using the same chromatographic conditions described above.
2.15. Statistical Analysis
- The results were subjected to analysis of variance (ANOVA) using SAS for Windows 9.0 (SAS Institute Inc., Cary, NC). Differences were considered significant at a 95% confidence level by using the Tukey test.
3. Results
3.1. Characterization of Isolates
- Bacterial isolates were obtained from commercial probiotic samples (Table 1). The morphological examination of the isolates showed straight rods with endospores. The bacteria grew aerobically, were strongly catalase positive and presented Gram-positive stain. Together with additional biochemical tests, the characteristics confirmed the isolates belong to the genus Bacillus [8].
3.2. Antimicrobial Activity
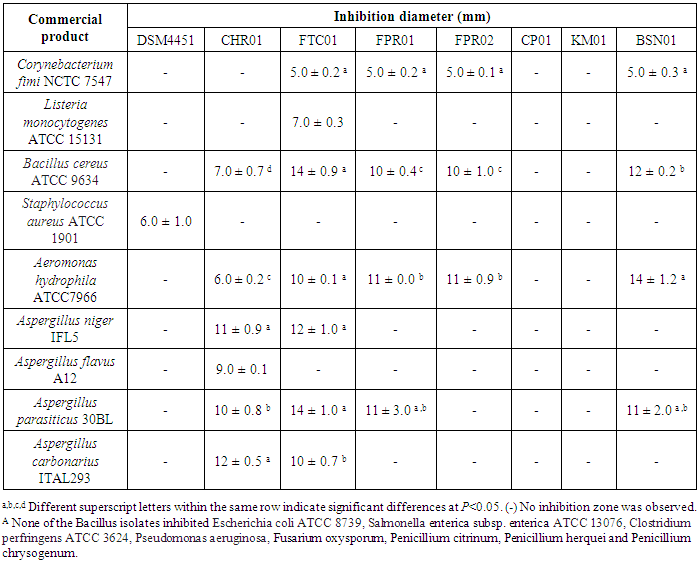
- The antimicrobial activity of Bacillus isolates was tested against a panel of indicator microorganisms, and the results are summarized in Table 2. The isolates CHR01 and FTC01 showed broad inhibitory spectra, followed by FPR01 and BSN01. The isolate FTC01 showed antibacterial activity against most of Gram-positive bacteria tested. The indicator strains C. fimi, L. monocytogenes, B. cereus and S. aureus were inhibited by at least one Bacillus isolate. In addition, it was observed that none of the isolates were able to inhibit the Gram-negative bacteria E. coli, S. enterica subsp. enterica and P. aeruginosa. In addition, only the culture supernatants of two Bacillus spp. tested (KM01 and CT01) showed no activity against the tested microorganisms. Among the fungi used as test organisms, only the Aspergillus species demonstrated sensibility to FTC01, CHR01, FPR01 and BSN01 isolates.
|
3.3. Antioxidant Activity
- The antioxidant potential of Bacillus isolates was tested by DPPH scavenging and reducing power assays. However, positive results were only observed by the last method. Reducing power assay is based on the ability of a compound to reduce the Fe3+/ferricyanide complex to the ferrous form (Fe2+). In the present study, the isolate KM01 showed the highest value (0.450), followed by FTC01, and BSN01 (Figure 1). The values were lower as compared to that observed for the standard antioxidant BHT.
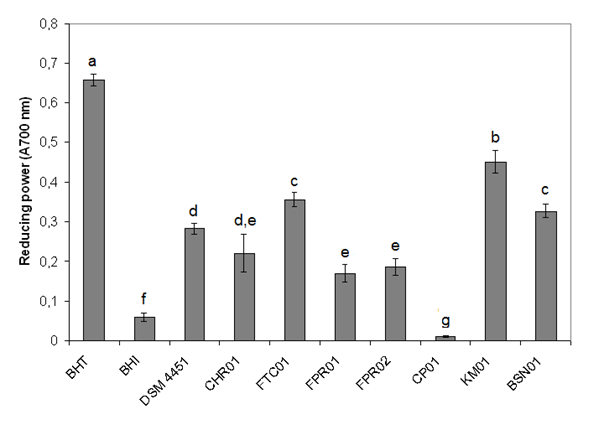 | Figure 1. Antioxidant activity of the Bacillus isolates. The strains were analyzed for their capability to reduce Fe3+/ferricyanide complex |
3.4. Bacterial Adhesion to Hydrocarbons (BATH) and Auto-Aggregation Tests
- In this work, BATH and auto-aggregation were studied as an index for adhesion properties. BATH values ranged from 0 to 56.4% adherence (Figure 2). Auto-aggregation of bacterial cells was determined after incubation at 15°C for 3 h, and values were expressed as percentage of adhesion (BATH, gray bars) or auto-aggregation (white bars). The highest value was observed for the strain DSM 4451. The isolates KM01 and FPR02 presented BATH values higher than 35%, while all other isolates showed very low values. Similar to the cell surface hydrophobicity, the self-aggregating activity ranged between 0 and 52.9%. The DSM 4451 strain showed the highest value of auto-aggregation, followed by CHR01 (Figure 2).
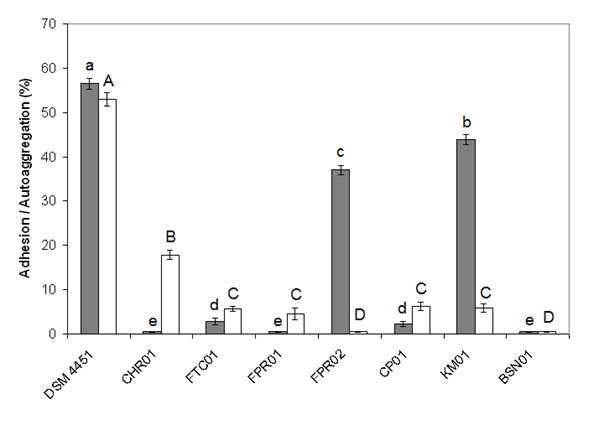 | Figure 2. Adhesion and auto-aggregation properties of the Bacillus isolates. Bacterial adhesion to hydrocarbon (BATH) test was conducted using xylene |
3.5. Bile and Acid Tolerance Test
- The Bacillus isolates were then tested for their capability to thrive in the presence of bile salts and acid pH. Regarding the presence of ox bile in the medium, the isolates DSM 4451, FTC01, CP01, KM01, FPR01 and BSN01 were tolerant, while FPR02 and CHR01 were found to be sensitive.Among the tested bacteria, isolates DSM 4451, CHR01, FTC01, FPR01 and CP01 showed more than 80% survival at pH 3 (Figure 3). After 2 h of incubation at pH 2, the FTC01 isolate was more tolerant to acid exhibiting 96% survivability. The FPR01 and FPR02 were the cultures showing lower resistance to pH 2.
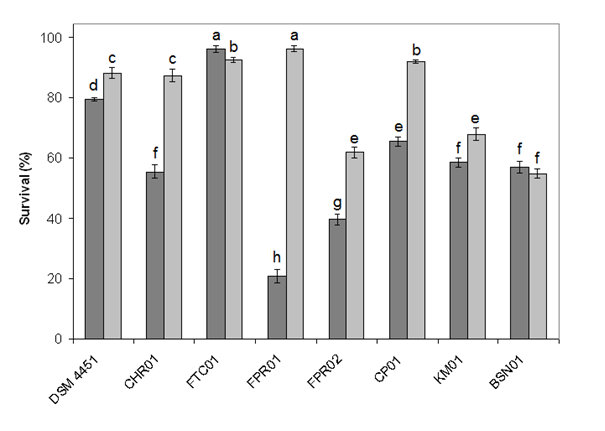 | Figure 3. Acid tolerance of Bacillus spp. isolates. The percentage of cell survival was determined after 2 h at pH 2.0 (gray bars) or pH 3.0 (light gray bars) |
3.6. Exopolysaccharide Production
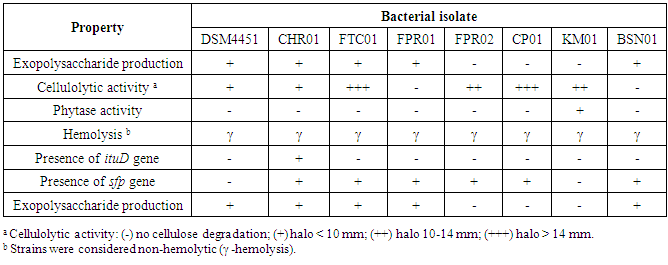
- The results of this study demonstrated that the isolates DSM 4451, CHR01, FTC01, FPR01 and BSN01 formed white ropy colonies due to the production of EPS, but this characteristic was not observed for FPR02, CP01 and KM01 (Table 3).
|
3.7. Haemolytic, Cellulolytic and Phytase Activity
- Excepting for isolates FPR01 and BSN01, the Bacillus spp. demonstrated cellulolytic activity, while only strain KM01 showed phytase activity (Table 3). None of the Bacillus tested showed hemolytic reaction (all γ-hemolytic).
3.8. Presence of sfp and ituD Genes and Surfactin Production
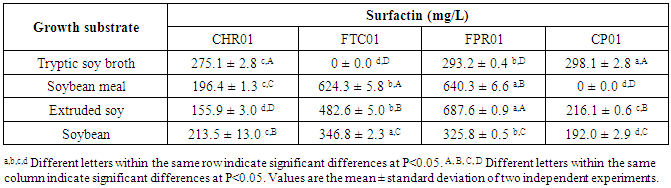
- According the results presented in Table 3, six Bacillus isolates contained the sfp gene and a single one (FTC01) presented ituD gene. For this reason, the production of surfactin was further investigated. Four strains (CHR01, FTC01, FPR01 and CP01) that showed the best in vitro results for probiotic potential were selected for evaluation of surfactin production on different type of soy substrates. Higher production of surfactin could be observed by Bacillus FPR01 in comparison with other Bacillus tested, independent of the substrate (Table 4). CHR01 and FPR01 were the isolates producing surfactin on all growth substrates.
|
4. Discussion
- This study was performed to investigate some in vitro probiotic characteristics of different Bacillus strains isolated from commercial formulations. The antimicrobial activity was initially accessed. The isolates CHR01 and FTC01 showed a wide range of inhibitory activity against the pathogenic microorganisms tested including mostly Gram-positive bacteria. Antagonistic activities of probiotic strains are important to prevent the infection or invasion of pathogenic bacteria. Members of the genus Bacillus are known to produce a wide range of antimicrobial substances, including peptide and lipopeptide antibiotics [34, 43]. Some strains of B. subtilis exhibit parallel antifungal and antibacterial properties and their antimicrobial spectra include both Gram-positive and Gram-negative bacteria [45]. B. subtilis PB6 growing in TSBYE broth produces a heat-stable anticlostridial factor, which was inactivated by proteases and showed an apparent molecular mass ranging from 960 to 983 Da [44]. However, inhibition of C. perfringens was not observed in this work when the isolate was grown in TSB. This corroborates that the production of antimicrobial substances by Bacillus spp. is a strain peculiarity and strongly influenced by environmental conditions [25, 46].The results of this study are in agreement with the fact that some Bacillus under study may exert their effect not by direct inhibition of pathogenic bacteria, but by competitive exclusion of undesirable microbiota [24, 40]. In addition, they can stimulate the immune system and modulate inflammatory response in colon mucosa [39, 40]. These beneficial effects may be associated with the control pathogenic bacteria previously observed in vivo for these strains.The CHR01 isolate demonstrated the best antifungal activity, especially against A. carbonarius. Significant inhibition of A. carbonarius was observed by Jiang et al. [22] when samples were treated with B. subtilis cultures, especially those subjected to cell-free culture treatments. The antifungal activity of five Bacillus strains isolated from fish intestines was tested against six different phytopathogenic fungi, and all strains caused growth inhibition of Fusarium spp., Bipolaris sorokiniana and Aspergillus spp., whereas the greatest inhibition occurred against Fusarium spp. [46]. The inhibition of toxigenic fungi can be relevant since the addition of such Bacillus to feed can be useful to reduce potential hazards caused by production of mycotoxins [47].The application of probiotic bacteria with antioxidant activity may increase food quality as well as its shelf life [31]. The DPPH has been widely used as a free radical to assess antioxidant substances and is a useful reagent for investigating the capture of free radical activity of the compounds [48]. Despite none of the bacteria tested in this study showed ability to scavenge the DPPH radical, several strains were capable to reduce the Fe3+/ferricyanide complex to the ferrous form Fe2+. Therefore, the reducing ability of Bacillus cultures indicates that they can act as electron donors in reducing the oxidized intermediates of oxidation processes and the reducing power probably contributes to the antioxidant activity [50].Bacterial aggregation between microorganisms of the same strain (auto-aggregation) and the ability to adhere to mucosa surfaces has been suggested to be an important property of bacterial strains used as probiotics [9]. In this work, the collection strain DSM 4451 showed consistent values for adhesion and auto-aggregation, while FPR02 and KM01 showed adhesion capability only. Another study on food-isolated Bacillus spp. with probiotic properties described values ranging 30-80% for adhesion and 70-100% for auto-aggregation [32]. The auto-aggregation is often correlated with adhesion, which is a prerequisite for colonization and infection of the gastrointestinal tract by many pathogens, whereas co-aggregation has been related to the ability to interact closely with pathogens [9].According to the guidelines on probiotic organisms reported by a joint FAO/WHO working group, two of the currently most widely used in vitro tests are the resistance to gastric acidity and bile compounds based on both survival and growth studies [16]. Bile tolerance studies are mostly carried out using 0.3% ox bile solution because of its similarity to human bile juice and because 0.3% is considered to be a crucial concentration to evaluate a bile-tolerant probiotic [32]. In this work, the Bacillus spp. were generally tolerant to bile salts, although two strains were susceptible. These results are consistent with those observed in other studies on isolates of B. subtilis and B. toyoi [11], and different strains of B. coagulans [21]. The survivability of Bacillus spp. in the gastric juice depends on their ability to tolerate low pH, which is an important probiotic characteristic [32]. The presence of Bacillus spp. tolerant to acidic environments has been previously suggested by Hyronimus et al. [21] testing Lactobacillus sporogenes, Bacillus laevolacticus and Bacillus racemilacticus and as result observed that only B. laevolacticus showed a significant survival rate at pH 2.5.The determination of hemolytic activity is considered a safety aspect for the selection of probiotic strains [16]. Maragkoudakis et al. [28] reported Lactobacillus strains producing shades of green zones around the colonies indicating α-hemolysis. Similarly, Ruiz-Moyano et al. [38] found positive α-hemolysis results among strains of Lactobacillus casei isolated from human feces and then discarded their use as potential probiotics. In this study, all strains were non-hemolytic, indicating that they are possibly safe bacteria for probiotic use.In this study, five strains were positive to EPS production. This ability is economically important since it can impart functional effects to foods and confer beneficial health effects [32]. Certain EPS are also required due to the physiological effects for the individual, contributing to the formation of bacterial aggregates and cell recognition and adhesion to the surface, facilitating the colonization in several ecosystems [13].The production of some enzymatic activities by potential probiotics can be beneficial in animal feed. In this research, six Bacillus strains demonstrated cellulolytic activity, being that the FTC01 and BSN01 strains presented better cellulolytic activity. These results are in agreement with Peixoto et al. [35], which reported that B. subtilis and Bacillus velesensis isolated from fish intestines showed capability to degrade cellulose. In addition, phytase (myo-inositol hexaphosphate hydrolase) is widely distributed in animal and plant tissues in several species of fungi and some bacteria. This enzyme hydrolyzes phytic acid to inositol and inorganic phosphate. Thus, phytases contribute to better digestion of certain components in monogastric animals (phosphorous utilization), also contribute to the decrease in levels of phosphorus excreted by the animals, with consequent reduction of pollution caused by excess phosphorus in the environment [33]. Despite phytase activity has been described for some Bacillus strains, only KM01 strain showed phytase activity under the conditions of this study.A growing interest on natural antimicrobial peptides produced by Bacillus spp. has been reported [1]. The lipopeptide surfactin has relevant features to health care and biotechnological applications, including antimicrobial and antitumor activities, inhibiting formation of fibrin clots, antiviral activity and biocontrol against phytopathogens and insects [34, 46]. In addition, surfactin is one of the strongest biosurfactants, and its role to modulate inflammatory response by regulating the eicosanoid and cytokine pathways has been suggested [39]. This study demonstrated that six Bacillus isolates contained the sfp gene. The genes responsible for producing surfactin are located in a Bacillus operon, and the sfp gene codes the enzyme 4'-phosphopantetheinyl transferase, characterized as essential for surfactin synthesis [25]. Similarly, Porob et al. [36] found sfp gene among 67% of Bacillus isolates from marine origin. Regarding the ituD gene, only the isolate FTC01 was positive. Hsieh et al. [20] tested 120 field strains of Bacillus spp., including several B. subtilis for presence of ituD gene. They found that 35% of the strains were positive.The strains CHR01, FTC01, FPR01, and CP01 produced surfactin growing on different types of soy substrates. Higher production of surfactin was observed by Bacillus FPR01, independently of the substrate. The synthesis of surfactin in B. subtilis has been extensively studied since it is the most important microorganism in the production of surfactin. However, some strains of Bacillus pumilus, Bacillus mojavensis, Bacillus licheniformis and Bacillus amyloliquefaciens are also able to synthesize surfactin [7]. The feasibility of using cheap agro-industrial byproducts as substrates to produce surfactin in solid-state fermentation by B. amyloliquefaciens has been investigated [51]. In that study, soybean flour resulted best surfactin production compared to other substrates (rapeseed meal, corn meal, wheat bran, bean cake and rice straw). Similarly, the addition of commercial B. subtilis probiotics to feed formulations containing soy substrates may also induce surfactin synthesis, resulting potential benefits to the host.
5. Conclusions
- The results from this study showed that Bacillus strains obtained from commercial formulations presented desirable in vitro properties for probiotics. Some strains showed a broad spectrum of antibacterial activity against Gram-positive bacteria and fungi. In addition, all the isolates presented negative hemolytic reaction. In general, the strains showed antioxidant activity and survivability in the presence of bile salt and low pH values. This will help the probiotic bacteria to reach the small intestine and colon and contribute to the balance of the intestinal microbiota.
ACKNOWLEDGEMENTS
- This work received financial support of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML