-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2018; 8(1): 9-18
doi:10.5923/j.microbiology.20180801.02

Isolation and Screening of Xylanolytic Fungi from Soil of Botanical Garden: Xylanase Production from Aspergillus flavus and Trichoderma viride
Olaoluwa Oyedeji, Abiodun Iluyomade, Isaiah Egbewumi, Abiodun Odufuwa
Department of Microbiology, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Olaoluwa Oyedeji, Department of Microbiology, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Xylanases are group of enzymes which bring about the hydrolysis of hemicelluloses. They have stimulated great interest due to their wide industrial and biotechnological applications. In this study, the isolation, screening and selection of xlanase-producing fungi from soil of botanical garden, Obafemi Awolowo University, Ile-Ife, Nigeria were carried out. Soil samples were collected aseptically from a depth of about 3 cm in the soil and transported in sealed cellophane bags to the laboratory where fungal isolation was carried out immediately. Characterization and identification of fungal isolates were performed using standard procedures and the isolates were then screened for xylanase production, under submerged fermentation. The effect of nutritional and environmental factors on xylanase production was determined with a view to optimize enzyme production from fungi. A total of twelve filamentous fungal species were isolated from the soil sample out of which Aspergillus flavus and Trichoderma viride were selected as the most appreciable xylanase-producers. A. flavus and T. viride exhibited xylanase yields of 28.17 ± 0.43 U/mL and 30.31 ± 0.68 U/mL, respectively, using a spore concentration of 1 x 106 spores/mL, at pH 4.0, temperature of 50°C and after 144 h of incubation. Maximum xylanase production was achieved by A. flavus with the use of xylan (28.06 ± 0.49 U/mL) as sole source of carbon and peptone (27.17 ± 0.25 U/mL) as sole source of nitrogen while optimum xylanase production was achieved by T. viride with the use of sucrose (22.37 ± 0.57 U/mL) as sole source of carbon and sodium nitrate (28.56 ± 0.46 U/mL) as sole source of nitrogen. As a result of the relatively high temperature and low pH conditions of xylanase production, both fungal species have high potentials as sources of xylanases for industrial and biotechnological applications.
Keywords: Xylan, Xylanases, Aspergillus flavus, Trichoderma viride, Submerged fermentation
Cite this paper: Olaoluwa Oyedeji, Abiodun Iluyomade, Isaiah Egbewumi, Abiodun Odufuwa, Isolation and Screening of Xylanolytic Fungi from Soil of Botanical Garden: Xylanase Production from Aspergillus flavus and Trichoderma viride, Journal of Microbiology Research, Vol. 8 No. 1, 2018, pp. 9-18. doi: 10.5923/j.microbiology.20180801.02.
Article Outline
1. Introduction
- Xylans (hemicelluloses) are the second most abundant natural polysaccharides, after celluloses, with which they form major components of plant cell wall (Collins et al., 2005). Xylan is a non-crystalline, complex polysaccharide consisting of β-D-xylopyranosyl units linked by β-1,4-glycosidic bonds (Sedlmeyer, 2014). This linear structure may be substituted with acetyl, glucuronosyl and arabinosyl side chains, forming interface between lignin and other polysaccharides in lignocelluloses (Sedlmeyer, 2014; Shanti et al., 2014). The nature and type of side chains vary depending on the botanical source and method of extraction (Habibi and Vignon, 2005). The most potential sources of xylan includes many agricultural crops such as straw, sorghum, sugar cane, corn stalks and cobs and hulls and husks as well as forest and pulp waste products from hardwoods and softwoods (Ebringerova and Heinze, 2000). Due to its structural complexity, several types of enzymes appear to be involved in the degradation of native xylan. The hydrolysis of the xylan backbone is accomplished by endoxylanases [EC 3.2.1.8] and β-xylosidases [EC 3.2.1.37] along with a variety of debranching enzymes, that is, α-L-arabinofuranosidases, α-glucuronidases and acetyl esterases (Shallom and Shoham, 2003; Collins et al., 2005). Endoxylanases, which attack the linear polyxylose chain are the most important type and have accordingly received the most attention (Sunna and Antranikian, 1997). They cleave the internal β-1,4- bonds in the xylan backbone at non modified residues, yielding different chain length substituted xylooligosaccharides (Zhang et al., 2007). β-xylosidase hydrolyse xylobiose and oligosaccharides to complete the depolymerization of xylan to xylose and probably relieves the end product inhibition of endoxylanase activity (Subramaniyan and Prema, 2000; Chavez et al., 2006). The other enzymes play important roles in the removal of side groups from polymeric xylan to create more sites for subsequent enzyme hydrolysis (Contreras et al., 2008). According to Markets and Markets Watch (2015), the global industrial enzyme market was valued at $ 4.2 billion in 2014 and is projected to grow at the rate of 7.0% to reach $6.2 billion worldwide by 2020. The driving force for industrial enzyme market are increase in investments, research and development of industrial enzymes, increase in demand for consumer goods and biofuel and the need for cost reduction and resource optimization. Recently, xylanases have been extensively studied due to their various biotechnological and industrial applications. They have numerous applications and biotechnological potentials for various industries including chemicals, fuels, food, bakery, brewery, animal feed, textile, pulp and paper industries (Zaldivar et al., 2001; Gupta and Kar, 2009; Buthelezi et al., 2011). An example is the bioconversion of lignocellulosic residues into their constituent sugars especially xylose which can then be converted into bioethanol and xylitol (Laxmi et al., 2008). Xylitol is a poly-alcohol, non-cariogenic sweetener suitable for diabetic and obese individuals and recommended for the prevention of osteoporosis, kidney and parenteral lesions (Polizeli et al., 2005). Xylanases are of great value in the baking industry for flour modification to improve the bread volume and crumb structure (Maat et al., 1992; Harbak and Thygesen, 2002; Romanowska et al., 2006; Khandeparker and Numan, 2008). Other industrial applications of xylanases are in the clarification of wines and juices (Hang and Woodams, 1997), biobleaching of wood pulp for paper production (Albert et al., 2011), and improvement of digestibility of livestock feedstock (Twomey et al., 2003). Xylanases are produced by microorganisms such as bacteria, fungi and yeasts (Polizeli et al., 2005). They are also found in marine algae, protozoans, crustaceans, insects, snails, seaweed and also seeds of plants during germination phase in the soil (Wong et al., 1988; Sunna and Antranikian, 1997). Filamentous fungi are industrially important producers of this enzyme due to extracellular release of xylanases, non-pathogenicity and high yield compared to yeasts and bacteria (Haltrich et al., 1996; Singh et al., 2003; Kar et al., 2006). Thermophilic fungi, because of the production of thermostable enzymes, have a wide commercial importance (Bruins et al., 2001; Monti et al., 2003). Thermophilic fungi can thrive at a temperature of 40-60°C. Such enzymes produced at high temperatures have physiological characteristics of biotechnological importance such as higher kinetic rates and thermostability and less chance of contamination and better storage capacity (Latif et al., 2006). Due to increased and widespread applications of xylanases in various fields, the discovery of new microbial sources capable of producing specific xylanase with desirable characteristics is important. Therefore, this study was designed to isolate, screen and select for filamentous fungi with appreciable xylanolytic activities. Optimization of culture conditions such as fermentation period, carbon and nitrogen sources, pH and temperature for higher production of xylanases, under submerged fermentation, was then carried.
2. Materials and Methods
2.1. Collection of Soil Sample
- Soil samples were collected from the botanical garden, Obafemi Awolowo University, Ile-Ife, Nigeria. The upper part of the soil and other soil debris were removed and the soil was dug to depth of about 3 cm from where the soil samples were collected aseptically using sterile hand gloves.
2.2. Isolation, Characterization and Identification of Fungi
- Serial dilution technique was used for isolation of fungi from soil in which 1.0 g of soil sample was serially transferred into 9 ml sterile distilled water and prepared dilutions up to 10-8 dilution and inoculated on potato dextrose agar medium containing 0.7% (w/v) beechwood xylan. The plates were incubated at 50°C for 6 days. Prominent fungal strains were subcultured onto fresh potato dextrose agar plates supplemented with 0.7% (w/v) beechwood xylan until pure cultures were obtained. They were subcultured to purity and were maintained on potato dextrose agar slants and stored at 4°C. Typical fungal isolates were identified by macroscopic characterization and microscopic examination using lactophenol cotton blue mount according to Barnett and Hunter (1972) and Domsch et al. (1980). They were identified by reference to standard identification manual.
2.3. Screening for Xylanolytic Fungi
- The isolated fungal strains were screened for their xylanolytic activities in fermentation medium containing beechwood xylan as sole carbon source, under submerged fermentation condition.
2.4. Production and Extraction of Enzyme
- Medium composition used for submerged fermentation (in g/L), according to the modified method of Mandels and Weber (1969), were K2HPO4, 0.5; KH2PO4, 2.0; KNO3, 9.9; MgSO4.7H2O, 0.1; FeSO4.7H2O, 0.1; MnSO4.7H2O, 0.01; ZnSO4.7H2O, 0.01. Beechwood xylan 1.0% (w/v) was added as the sole source of carbon. Fermentations were performed in 250 mL Erlenmeyer flasks containing 100 mL of the culture medium. Standardized spore suspensions (1.0 x 106 spores/mL) were used to inoculate 100 mL of pre-sterilized basal medium. The fermentation culture was incubated at 50°C for 144 h. After incubation, the whole content of the flasks were centrifuged at 5000 rpm for 10 min at 4°C using the refrigerated ultracentrifuge. This was then filtered through glass microfiber filter (Whatman GF/A, UK) and the crude supernatant used for enzymatic assays. Experiments were carried out in triplicates and results were expressed as average values.
2.5. Xylanase Assay
- Xylanolytic activity was determined by measuring the release of reducing sugar D-xylose in a reaction mixture of 0.9 mL of the crude supernatant and 0.1 mL of 1.0% (w/v) oatspelt xylan (Sigma, St Louis, MO, USA) in 0.05 M citrate phosphate buffer (pH 5.0) incubated at 50°C for 15 min (Bailey et al., 1992). The reaction was terminated by addition of 1.5 mL of 3, 5-dinitrosalicylic acid (DNSA) reagent (Miller, 1959). Each tube was incubated for 5 min in a boiling water bath and then cooled rapidly. The xylanase activity of the reaction mixture was measured against a blank sample at wavelength of 540 nm. Xylanase activity was determined from a calibration curve constructed with varying concentrations of D-xylose (Sigma, St. Louis, MO, USA). One unit (U) of xylanase activity is defined as the amount of enzyme that liberated 1.0 µmol of D-xylose per milliliter per min under the assay conditions.
2.6. Optimization of Xylanase Production
- The effect of different cultural and environmental factors on enzyme production from the selected xylanolytic fungi was determined.
2.6.1. Effect of Carbon Sources on Xylanase Production
- The effect of different carbon sources such as glucose, soluble starch, sucrose, lactose, maltose, galactose, sorbitol and xylose, at 1.0% (w/v), on xylanase production from the selected fungi were determined. After inoculation with 1.0 ml standardized spore suspensions of the isolates, the flasks were incubated at 50°C for 144 h. At the end of incubation, the culture supernatants were assayed for enzyme activities using the method stated above.
2.6.2. Effect of Nitrogen Sources on Xylanase Production
- The effect of various nitrogen sources, at 5.0 g/L, on xylanase production was determined by supplementing the fermentative medium with different nitrogen sources (ammonium nitrate, ammonium sulphate, sodium nitrate, peptone, potassium nitrate and yeast extract). Fermentation was carried out at 50°C for 144 h. The cell-free supernatant obtained was assayed for xylanase activity as stated above.
2.6.3. Effect of pH and Temperature on Xylanase Production
- The effect of initial pH on xylanase production was determined by varying the pH values of the fermentation medium from 3.5 to 8.0. The influence of temperature on xylanase production was also determined by varying the temperature of incubation from 30°C to 70°C. Submerged fermentation was carried out for 144 h. The cell-free supernatant obtained was assayed for xylanase activity as stated above.
2.6.4. Effect of Fermentation Period on Xylanase Production
- The influence of fermentation periods, up to 168 h, using optimal conditions established previously, was studied. The xylanase activities in fermentation media with 1.0% (w/v) beechwood xylan as sole source of carbon were measured at 24 h intervals throughout the 168 h incubation period.
2.6.5. Effect of Inoculum Size on Xylanase Production
- The influence of various inoculum sizes ranging from 1.0 mL to 4.0 mL standard spore suspensions (1.0 x 106 spore/mL) on xylanase production was studied. Fermentation media inoculated with the different inoculum concentrations were incubated at 50°C and assayed for xylanase production at the end of 144 h period.
2.7. Statistical Analysis
- The data obtained were expressed as mean ± standard deviation of triplicate determinations for all experiments.
3. Results
3.1. Isolation, Characterization and Identification of Fungi
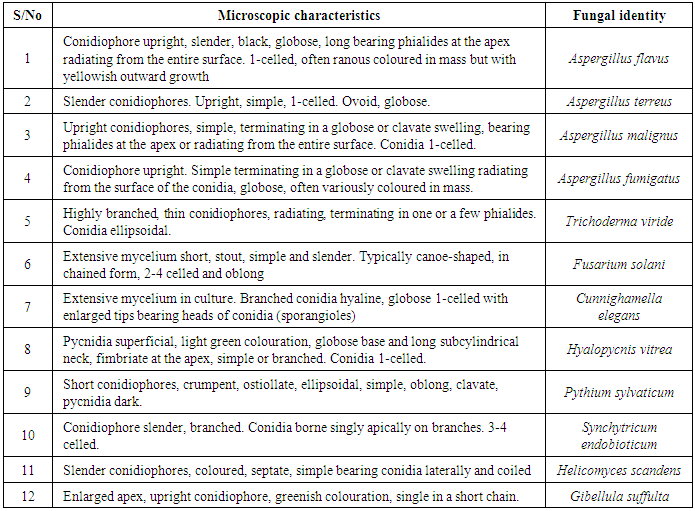
- Based on morphological, cultural and microscopic characterization, the typical fungal isolates from soil of botanical garden were identified as strains of Aspergillus flavus, Aspergillus malignus, Aspergillus terreus, Aspergillus fumigatus, Trichoderma viride, Fusarium solani, Cunninghamella elegans, Hyalopycnis vitrea, Synchyticum endobioticum, Pythium sylvaticum, Helicomyces scandens and Gibellula suffulta (Table 1).
|
3.2. Screening of Fungi for Xylanolytic Activity
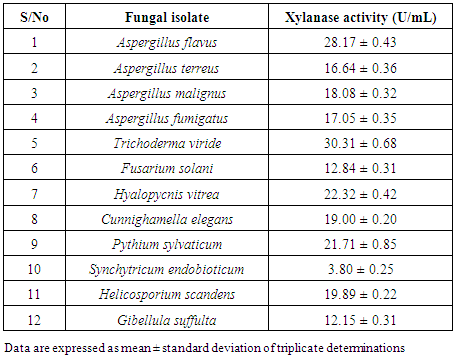
- The twelve fungal strains isolated from soil of botanical garden were screened for their xylanolytic activities under submerged fermentation condition using beechwood xylan as the sole source of carbon. All the isolates exhibited the ability for xylanase production to different extent. Aspergillus flavus (28.17 ± 0.43 U/mL) and Trichoderma viride (30.31 ± 0.68 U/mL) exhibited the highest xylanase activities (Table 2). Therefore, these two were selected for subsequent studies.
|
3.3. Influence of Carbon Sources on Xylanase Production
- A. flavus utilized different carbon sources, in the fermentation medium, for xylanase production with the maximum enzyme yield being observed with the use of beechwood xylan (28.06 ± 0.49 U/mL) as carbon source followed by sorbitol (16.80 ± 0.32 U/mL) (Figure 1). However, the use of xylose as the carbon source produced the least xylanase activity of 3.82 ± 0.30 U/mL. For Trichoderma viride, maximum xylanase yield of 22.37 ± 0.57 U/mL was observed with the use of sucrose as carbon source followed by the use of beechwood xylan (19.63 ± 0.18 U/mL). However, the use of xylose as carbon source produced the least enzyme (2.87 ± 0.38 U/mL) (Figure 1).
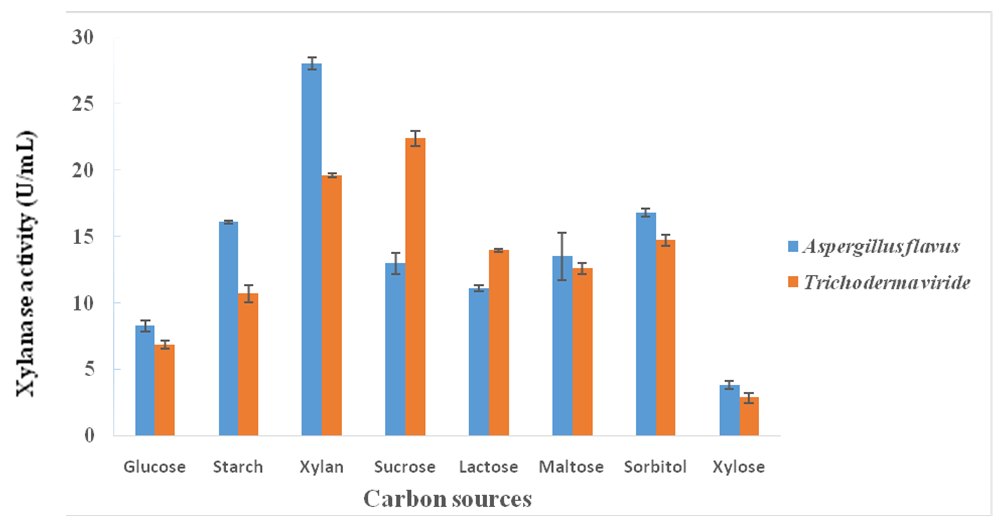 | Figure 1. Effect of different carbon sources on xylanase production from A. flavus and T. viride |
3.4. Influence of Nitrogen Sources on Xylanase Production
- Among the nitrogen sources used for xylanase production from A. flavus, peptone produced the maximum yield (27.17 ± 0.25 U/mL) followed by ammonium nitrate (25.67 ± 0.52 U/mL) while sodium nitrate produced the least enzyme yield (8.03 ± 0.18 U/mL) (Figure 2). T. viride produced maximum xylanase yield (28.56 ± 0.46 U/mL) with the use of sodium nitrate as nitrogen source, followed by the use of peptone (28.20 ± 0.28 U/mL), while ammonium sulphate produced the least enzyme yield (9.85 ± 0.27 U/mL (Figure 2).
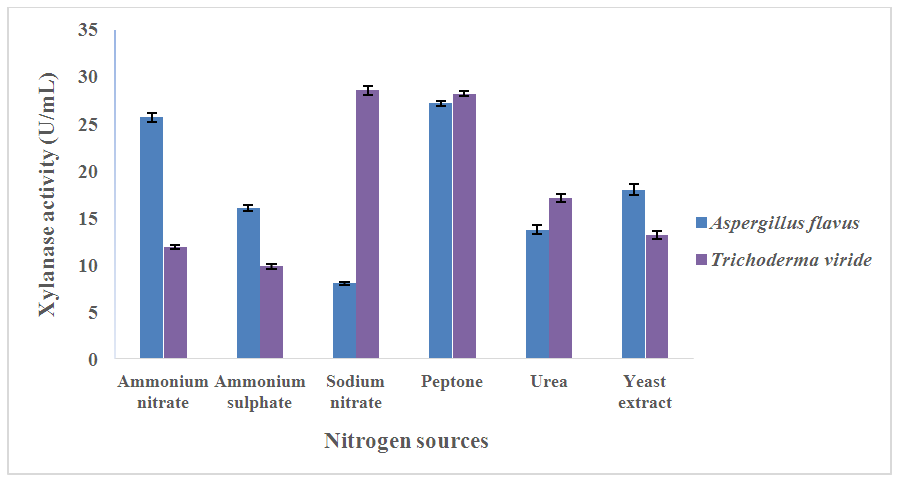 | Figure 2. Effect of different nitrogen sources on xylanase production from A. flavus and T. Viride |
3.5. Influence of pH and Temperature on Xylanase Production
- Figure 3 shows the influence of pH on xylanase production from A. flavus and T. viride. Optima xylanase activities and hence production were observed at the pH value of 4.0 in the case of both A. flavus (29.46 ± 0.34 U/mL) and T. viride (29.06 ± 0.34 U/mL). pH values above this resulted into steady decrease in xylanase production from both fungi (Figure 3). Both fungi attained maximum xylanase production at the temperature of 50°C; A. flavus (27.95 ± 0.34 U/mL) and T. viride (24.94 ± 0.26 U/mL) (Figure 4). Above this temperature, enzyme production decreased steadily in both cases (Figure 4).
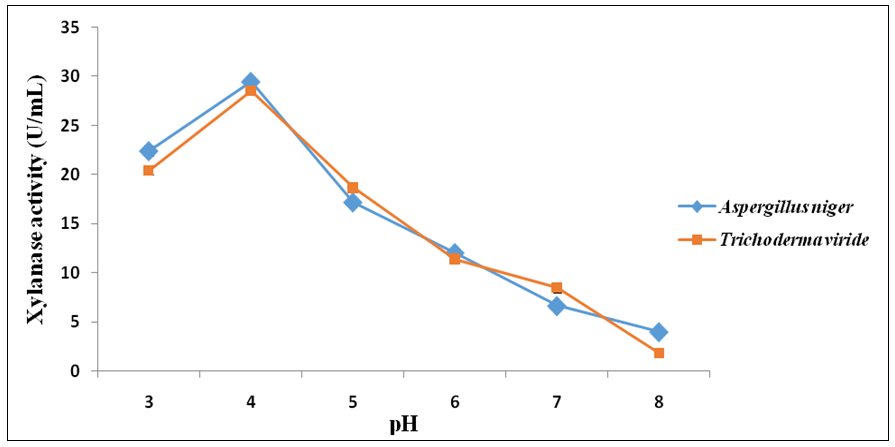 | Figure 3. Effect of pH on xylanase production from A. flavus and T. viride |
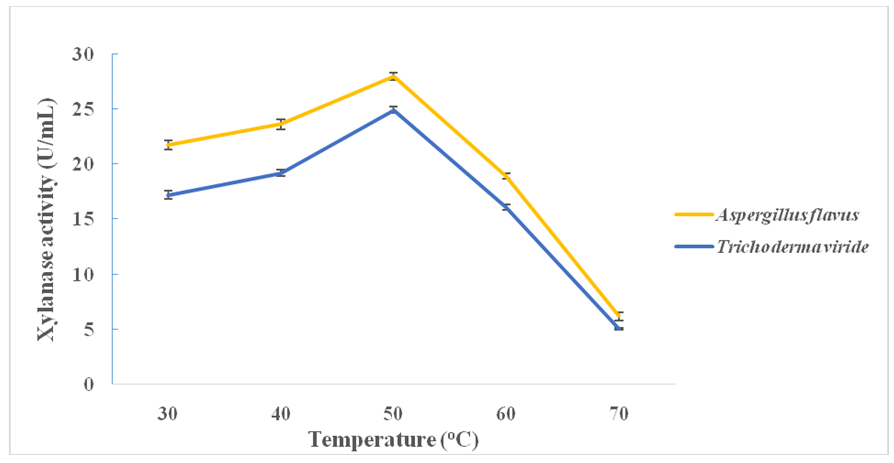 | Figure 4. Effect of temperature on xylanase production from A. flavus and T. viride |
3.6. Effect of Fermentation Period on Xylanase Production
- Fermentation time profile showed considerable variation in xylanase production from both fungal isolates. The xylanase activity increased with fermentation period until it reached the maxima 30.19 ± 0.79 U/mL and 32.91 ± 0.18 U/mL, in the case of A. flavus and T. viride, respectively, after 144 h fermentation period. Beyond this period, there was a steady decline in xylanase activity in both cases (Figure 5).
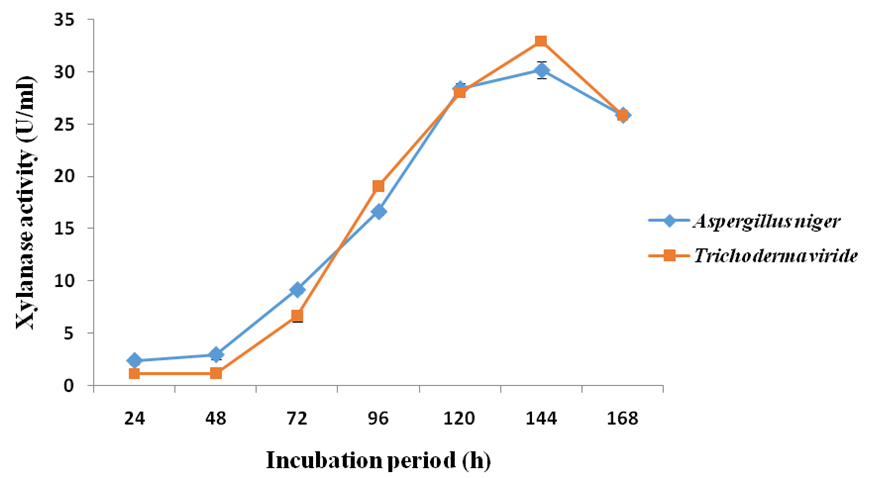 | Figure 5. Effect of incubation period on xylanase production from A. flavus and T. viride |
3.7. Effect of Inoculum Concentration on Xylanase Production
- Maximum xylanase production from each of A. flavus (26.55 ± 0.39 U/mL) and T. viride (25.94 ± 0.23 U/mL) was obtained with the use of 1.0 mL (1.0 x 106 spores/mL) fungal inoculum concentration. A gradual decrease in enzyme production was observed with the use of increasing inoculum concentrations of fungal spores from 2.0 mL (2.0 x 106 spores/mL) to 4.0 mL (4.0 x 106 spores/mL), in both cases (Figure 6).
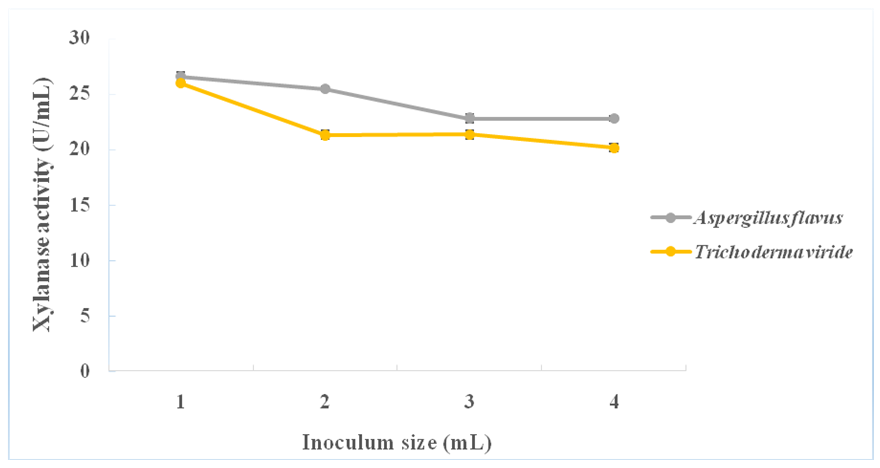 | Figure 6. Effect of inoculum size on xylanase production from A. flavus and T. viride |
4. Discussions
- Filamentous fungi with xylanase-producing ability were isolated from soil of botanical garden. Forest soils are a rich source of hemicellulose (xylan), and the xylan and xylose-utilizing microflora for exploitation in different industrial processes (Torres et al., 2013). Many workers have characterized fungal isolates from garden soils in which diverse species were obtained (Tallapragada and Venkatesh, 2011). Xylanases are important industrial enzymes which depolymerizes xylan molecules into xylose units (Garg et al., 2011). They have wide applications with potentials for use in the food processing, beverage, livestock feed, paper and pulp, detergent and textile industries as well as in the conversion of agricultural (lignocellulosic) biomass into products with commercial value (Twomey et al., 2003; Garcia et al., 2001; Romanowsky et al., 2006; Albert et al., 2011). With the increasing demand for alternative liquid fuels worldwide, the enzyme is used for enzymatic hydrolysis of lignocellulosic biomass in bioethanol production process. In the present study, a total of twelve filamentous fungal isolates were collected from soil of botanical garden and screened for their xylanase activity under submerged fermentation condition. Aspergillus flavus and Trichoderma viride exhibited relatively better xylanolytic activities and were therefore selected for further studies. Most fungal species are known for the secretion of xylanase but species belonging to the genera Aspergillus and Trichoderma have been reported to produce the enzyme on an industrial scale (Fengxia et al., 2008).Production of microbial enzymes is dependent upon various nutritional and cultural factors such as initial pH, temperature, carbon and nitrogen sources and inoculums sizes and hence these were studied in order to optimize xylanase production from the selected fungi. The best carbon sources for xylanase production from A. flavus and T. viride were found to be xylan and sucrose, respectively, while the use of xylose as source of carbon produced least enzyme in both cases. Induction of the synthesis of xylan-degrading enzymes by xylanolytic organisms cultured with xylan as carbon source is well documented (Collins, 2005). However, since xylan is unable to enter the microbial cell, it has been suggested that low molecular weight degradation products of xylan hydrolysis penetrate into the cells and induce the production of hydrolytic enzymes (Haltrich et al., 1996; Tallapragada and Venkatesh, 2011). Thus xylanases may also be induced by monosaccharides and dissacharides. Several substances have been indicated as suitable carbon sources for xylanase producing microorganisms including oat spelt xylan (Saha, 2002), birchwood xylan (Duarte et al., 1999) and wheat arabino-xylan (Bataillon et al., 2000). Low level xylanase production in the presence of xylose in this study could be attributed to repression of the synthesis of xylanolytic enzymes involved in the utilization of xylan by easily metabolizable xylose as demonstrated in many organisms (Bindu et al., 2006).Organic nitrogen source peptone gave the maximum yield of xylanase from A. flavus followed by ammonium nitrate while sodium nitrate was the best nitrogen source for xylanase production from T. viride followed by peptone. Gupta et al. (2009) also reported peptone to be the best source of organic nitrogen for the production of xylanase from Aspergillus niger and Fusarium solani. However, yeast extract was observed to be the best nitrogen source for xylanase production from Aspergillus sp. RSP-6 (Bakri et al., 2008).pH is an important cultural or environmental parameter that determines growth rate and has major effect on levels of enzyme production by microorganisms. Optimum pH for xylanase production from both isolates was found to be 4.0. The highest endoxylanase activity was also detected at pH 4.0 for the enzyme production from Aureobasidium pullulans SN090 (Nasr et al., 2013). Whilst xylanase production at pH range 7 - 8 has been reported for several fungi (Xiong et al., 2004; Tallapragada and Venkatesh, 2011; Nour El-Dein et al., 2014) and Bacillus subtilis BS04 (Irfan et al., 2016), xylanase production at acidic pH have been indicated by previous researchers. Maxima xylanase production were achieved from strains of Penicillium sp. at pH 5.0, Trichoderma sp. T-1 at pH 5.5 (Mohan et al., 2011) and from A. terreus UL at pH 6.0 (Chidi et al., 2008). According to Bailey et al. (1993), the optimal pH for xylanase production depends not only on the fungal strain considered, but also on the nature of the carbon source utilized in the cultivation medium.The fermentation temperature has marked effect on the level of xylanase production as it plays important role in the metabolic activities of microorganisms (Seyis and Aksoz, 2003). The optimum temperature of 50oC was observed for xylanase production from both fungal strains. A decrease in xylanase production was observed below and above this optimum. This result was similar to the reported optimum temperature of 50°C observed for xylanase production from Streptomyces sp. K37 (Nour El-Dein et al. 2014) and 55°C for a strain of Bacillus sp. (Buthelezi et al., 20011). However, lower levels of temperature of 25°C and 30°C were observed for xylanase production from strains of Trichoderma sp. (Pang et al., 2006) and A. niger (Simoes et al., 2009), respectively. Optimum temperature for enzyme production depends on strain variation of the microorganisms (Gautam et al., 2010).The time course of xylanase production from the selected fungi were investigated and maximum production was observed after 144 h fermentation period in the case of both fungi. Further incubation beyond this period led to decreasing levels of enzyme production, probably due to increased level of toxic waste metabolites and decreasing levels of nutrients in the fermentation medium leading to decreased growth and enzyme production. This result is similar to those reported for xylanase production from strains of T. viride (Simoes et al., 2009) and A. niger (Tallapragada and Venkatesh, 2011).The use of the inoculum concentration 1.0 x 106 spores/mL resulted in the maximum xylanase production from each of A. flavus and T. viride. Higher spore concentrations produced decreased levels of xylanase in the culture medium in both cases. Gawande and Kamat (2000) and Seyis and Aksoz (2004) observed best xylanase activities using inocula concentration of 1.5 x 106 spore/mL and 1.0 x 106 spore/mL, respectively, of different strains of Trichoderma sp. According to Brown and Zainudeen (1978), very high concentrations of fungal spores lead to a decrease in the specific velocity of oxygen consumption, which affects fungal metabolism and hence enzymatic activity.
5. Conclusions
- Results of this study has revealed that Aspergillus flavus and Trichoderma viride, isolated from soil of botanical garden, Obafemi Awolowo University, Ile-Ife, Nigeria exhibited unique characteristics of thermostability and the ability for appreciable extracellular xylanase production. Optimal cultural factors for xylanase production from the two selected fungi were a fermentation period of 144 h and optima pH and temperature of production 4.0 and 50°C, respectively. Maximum xylanase production was achieved from A. flavus with the use of xylan as sole source of carbon and peptone as sole source of nitrogen while optimum xylanase production was achieved from T. viride with the use of sucrose as sole source of carbon and sodium nitrate as sole source of nitrogen. As a result of the relatively high temperature and low pH conditions of xylanase production, both fungal species have high potentials as sources of xylanases for industrial and biotechnological applications.
ACKNOWLEDGEMENTS
- Authors appreciate the laboratory staff of the Department of Microbiology, Obafemi Awolowo University for provision of laboratory reagents and facilities for carrying out this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML