-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(4): 99-106
doi:10.5923/j.microbiology.20170704.03

Isolation and Genotypic Characterization of Microbial Contaminants in Unpasteurized Fresh Juices Sold in Port Harcourt, Rivers State, Nigeria
Odu Odu Ngozi Nma , Ihuoma Ahaotu , Ferdinand Ugbong
Department of Microbiology, University of Port Harcourt, Nigeria
Correspondence to: Odu Odu Ngozi Nma , Department of Microbiology, University of Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

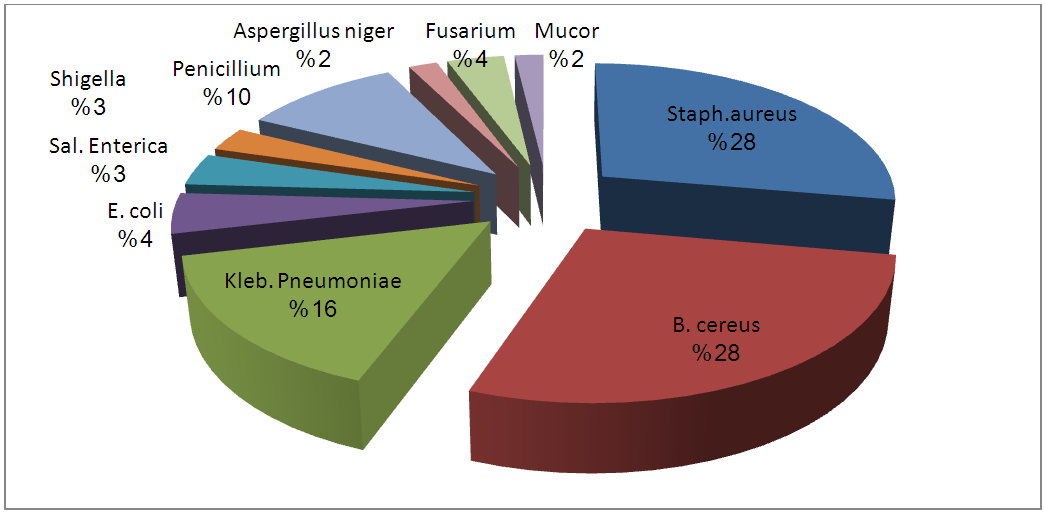
In recent years, the demand for freshly produced fruit juices has increased due to the nutritional benefits. This study was aimed at evaluating the microbial quality of unpasteurized juices sold in Port-Harcourt, Rivers State, Nigeria and to carry out genotypic identification of the pathogens. One hundred and thirty-five (135) samples of freshly produced orange (45), watermelon (45) and pineapple (45) juices were purchased from 3 different locations in Port Harcourt. Bacterial isolates were characterized using genotypic methods through PCR amplification of specific region of 16sRNA. The mean heterotrophic count ranged from 3.0 x 103 to 5.1 x 104 cfu/ml; mean coliform count ranged from 2.0 x 102 to 3.0 x 104 cfu/ml while mean fungal count ranged from 1.4 x 102 to 2.0 x 102 cfu/ml. A total of 6 bacterial genera were isolated and identified as Staphylococcus aureus (28%); Bacillus cereus (26%); Klebsiella pneumonia (14%); Escherichia coli (5%); Salmonella enterica (4%) and Shigella sp. (3%). The fungal isolates were identified as Penicillium sp (8%), Aspergillus sp. (5%), Fusarium sp. (4%) and Mucor (3%). The high microbial load and presence of enteric pathogens indicate poor sanitary quality of products. In order to obtain maximum benefits from the fruit juices, fruit juice vendors need to be educated on good manufacturing practices (GMP) and HACCP technique. Regulatory bodies should also ensure compliance to guidelines already established.
Keywords: Unpasteurized, Fruit juices, Genotypic, Enteric Pathogens, Sanitary Quality
Cite this paper: Odu Odu Ngozi Nma , Ihuoma Ahaotu , Ferdinand Ugbong , Isolation and Genotypic Characterization of Microbial Contaminants in Unpasteurized Fresh Juices Sold in Port Harcourt, Rivers State, Nigeria, Journal of Microbiology Research, Vol. 7 No. 4, 2017, pp. 99-106. doi: 10.5923/j.microbiology.20170704.03.
Article Outline
1. Introduction
- Fruit juice can be defined as fruit extracts, not treated but treatable, ready for immediate consumption which can be obtained by manually operated machine from healthy fruits. In Nigeria, fruits such as orange, watermelon and pineapple are cultivated and consumed as snacks but could also be cultivated for the production of fruit juices (Mensah et al., 2010; Odu and Njoku, 2010). Fruit and vegetables are widely exposed to microbial contamination through contact with soil, dust, water and by handling at harvest or during postharvest processing. They therefore harbor a diverse range of microorganisms including human and plant pathogens (Dunn et al., 1995; Beuchat, 2002; Carmo et al., 2004).General composition of fruit juice depends largely on the stage of maturity, method of harvesting and origin of the fruit. Fruit juice contains carbohydrate in form of sugars, hemicelluloses, cellulose polymers. Protein is also present, though in small quantity especially in oily fruits (Anon, 2012). Speck (2010) reported the presence of acids such as citric, tartaric, malic, lactic, acetic and ascorbic acids especially in unripe fruits. Acids play very important role in determining the taste of fruit juices due to the appropriate balancing of sugar content. Sugar is one of the important constitutes of fruit juices because of it high nutritive quality and also as a result of its property as sweeteners with good aroma (Speck, 2010).In a study carried out by Odu and Njoku (2010), they observed that fruit juices are important sources of water soluble vitamins such as (vitamins A and C) and fat soluble vitamins like vitamins B1, B2 B12 and folic acid. Fruit juice is very rich in calcium and potassium. Medical experts recommend the consumption of fruit juices rich in essential nutrients such as vitamin C, calcium and riboflavin to prevent the risk of constipation and other health issues such as multiple sclerosis (Arthey and Ashurst, 2001). Depending on the cultivar (species) moderate amounts of phytochemicals such as tannins and phenols, flavonoids and saponins have been reported (Anon, 2012).In South-South Nigeria, processing/vending of fresh unpasteurized juice is a very common small scale enterprise which is on the increase due to high demands. Sequel to the above, this study was undertaken to determine the microbial load of unpasteurized juices sold in Port-Harcourt, Rivers State, Nigeria and to genotypically identify the associated pathogens.
2. Material and Methods
- Study LocationThe study area, Port-Harcourt, is situated in the South-South geopolitical zone of Nigeria and lies between latitude 4°15′ and 5°45′ North, and longitude 6°20′ and 7°35′ East of the equator. Bounded at the North by Imo and Anambra States, South by the Atlantic Ocean, East by Akwa-Ibom and Abia States, and the West by Bayelsa and Delta States.Sample SitesOrange, pineapple and watermelon juices were purchased from three different locations which included GRA phase 1, Choba and Mile 3 in Port Harcourt, Rivers State, Nigeria.Collection of Samples One hundred and thirty-five (135) samples of freshly produced orange (45), pineapple (45) and watermelon (45) juices, were each collected in 50 ml bottles as packaged by the vendors and transported to the laboratory in an ice box maintained at 4°C and analyzed within 1 h.Media and ReagentsNutrient agar (TM, India), MannitolSalt Agar (Lab M, UK), MacConkey agar (Lab M, UK), Eosine Methylene Blue agar (Lab M, UK), SalmonellaShigella Agar (Micro master, India) Potato Dextrose Agar (Lab M, UK) and Peptone water (TM, India). All media used were prepared according to manufacturer’s instructions. All reagents used were of analytical standard.Enumeration, Isolation and Identification of Bacteria and Fungi IsolatesMicrobiological analysis of unpasteurized fresh fruit juices was done according to the method described by Odu and Njoku (2010). Ten milliliter (10 ml) of each sample was transferred to 90 ml sterile peptone water and mixed thoroughly to make the first dilution. It was subsequently diluted to 10-3 dilution. The diluted samples were plated out on the various media using spread plate technique. All the inoculated plates except acidified PDA plates were incubated at 37°C for 24 - 48 h. The acidified PDA plates were left at room temperature (25°C – 27°C) for 24 - 72 h. Thereafter, plates with growth between 30 and 300 colonies were selected and counted. Colonial morphology were also recorded. Colony counts were expressed as colony forming unit per milliliter (cfu/ml).Purification of ColoniesColonies were purified by repeated sub-culturing and then preserved on agar slant at 4°C for further identification and characterization.Identification of IsolatesThe isolates were identified based on cultural and biochemical characteristics using Bergey’s Manual of Determinative Bacteriology (Jolt et al., 1994). Bacteria isolates were characterized using biochemical and molecular methods while Fungi were identified based on macroscopic and Microscopic characteristics with special reference to standard identification keys and Atlas (Santos et al., 1988; Harrigan and McCance, 1990).Determination of PH of test samplesThe PH of the various fruit juices were determined in duplicates using a PH meter (model RS232 Jenway, UK). Before use, the PH meter was calibrated using PH 4 and PH 7 solutions. The electrode was dipped into a 5ml juice contained in a 20ml beaker. The average readings from these juices were recorded.
3. Molecular Characterization
- Molecular characterization of pathogenic and spoilage organisms isolated from unpasteurized fresh fruit juices sold in Port Harcourt was done using the Jena Bioscience bacteria DNA preparation kit as described by Abdussalam and Kafertein (2012).Amplification of specific region of DNA strand such as 16 SrRNA was carried out using polymerase chain reaction (PCR). A mix reaction comprising of 25 μl primers was subjected to PCR and the concentration of the reaction was reduced from 5 x with IX blend mix buffer. The mix reaction was made up with MgCl2 (1.5 mM), each of the nucleoside triphosphate (200 μm), each primer (25 m POI), enzyme for proof reading, DNA polymerase and sterile distilled water. A series of repeated temperatures called thermal cycling were used for denaturation of DNA hydrogen bond at 95°C for 15 min.Amplification of DNA strand was done at 95°C for 30 sec and elongation of primers at 72°C for 10 min. Exactly 1.5% agarose gel electrophoresis with known wavelength was used for size separation at 80 V for 1 h 30 min and DNA bands were viewed using ethidium bromide staining. DNA molecular weight used was 100 bp DNA ladder. The sequences were then purified using Exo sap and package for DNA Sanger sequencing. ABI sequencing analysis software (version 5.2) was used to analyze the data generated for gene sequencing.
4. Results
- Heterotrophic Bacteria CountHeterotrophic bacteria counts of unpasteurized fresh orange, watermelon and pineapple juices are presented in Fig. 1. Unpasteurized orange juice had counts ranged from 4.2 x 103 to 4.9 x 104 cfu/ml; 3.2 x 103 to 5.1 x 104 cfu/ml(unpasteurized fresh watermelon juice) while 3.2 x 103 to 6.1 x 104cfu/ml (unpasteurized fresh pineapple juice).
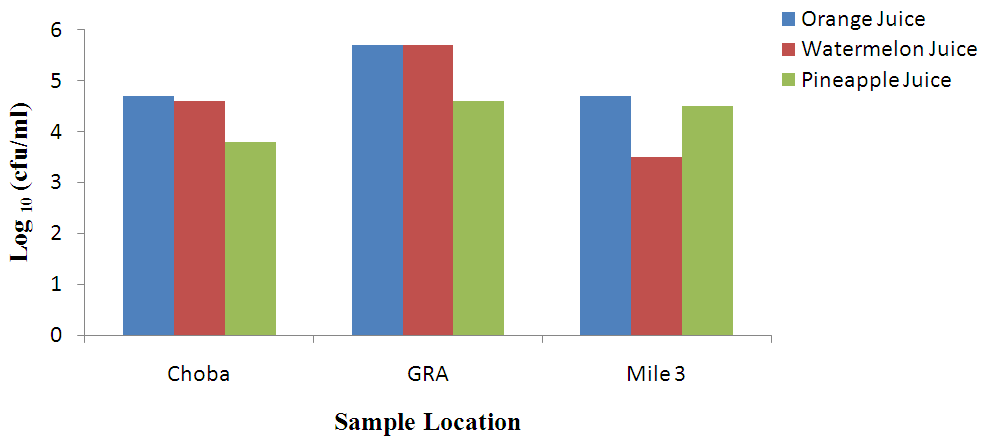 | Figure 1. Heterotrophic bacteria count (cfu/ml) of unpasteurized fresh fruit juices in Choba, Government Reserved Area Phase 1 (GRA) and Mile 3 Market (Mile 3) |
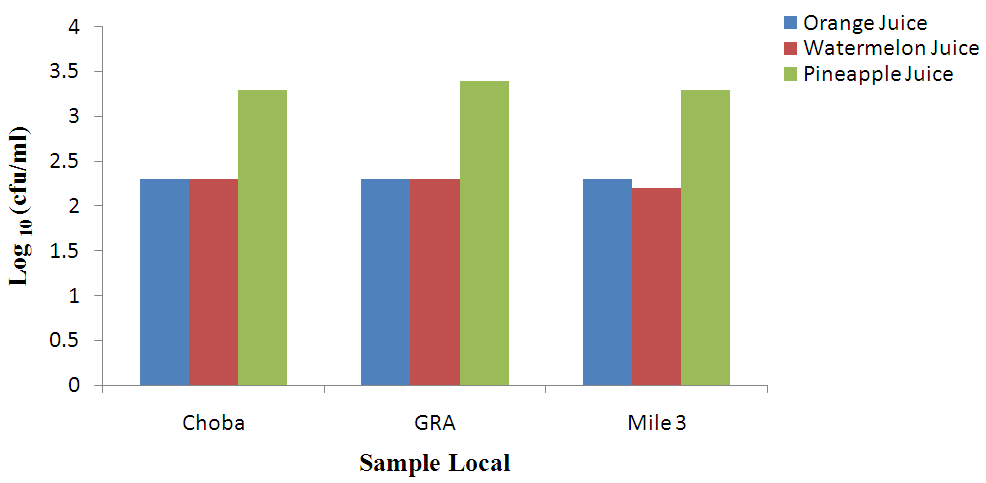 | Figure 2. Coliform count (cfu/ml) of unpasteurized fresh fruit Juices in Choba, Government Reserved Area Phase 1 (GRA) and Mile 3 Market (Mile 3) |
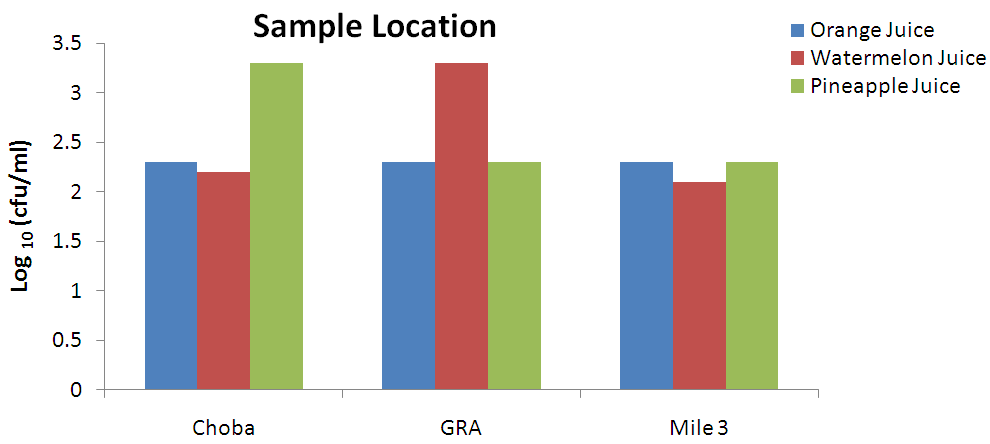 | Figure 3. Fungal count (cfu/ml) of unpasteurized fresh fruit juices in Choba, Government Reserved Area Phase 1 (GRA) and Mile 3 Market (Mile 3) |
 | Figure 4. Percentage Occurrence of Isolates from Unpasteurized Fresh Orange Juices Sold in Port –Harcourt |
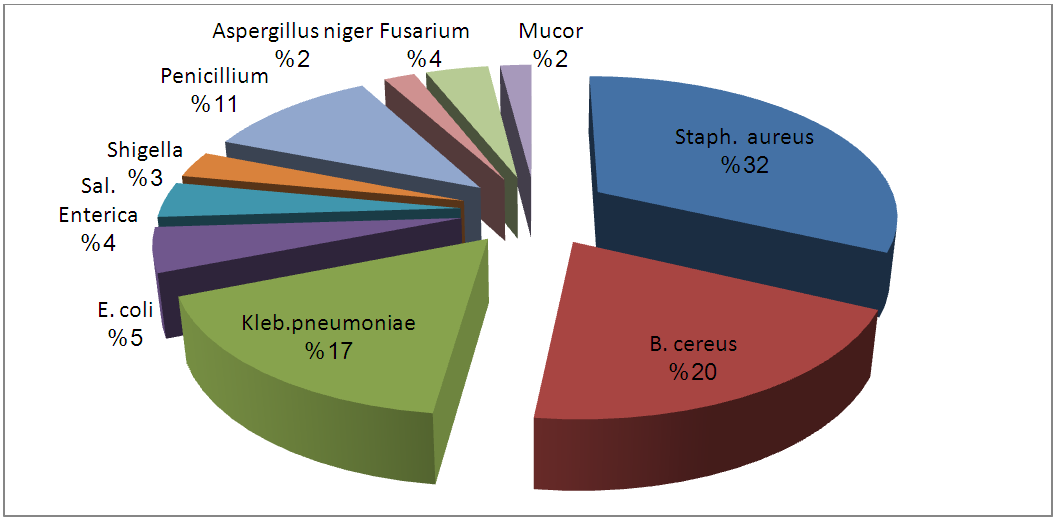 | Figure 5. Percentage Occurrence of Isolates from Unpasteurized Fresh Watermelon Sold in Port- Harcourt |
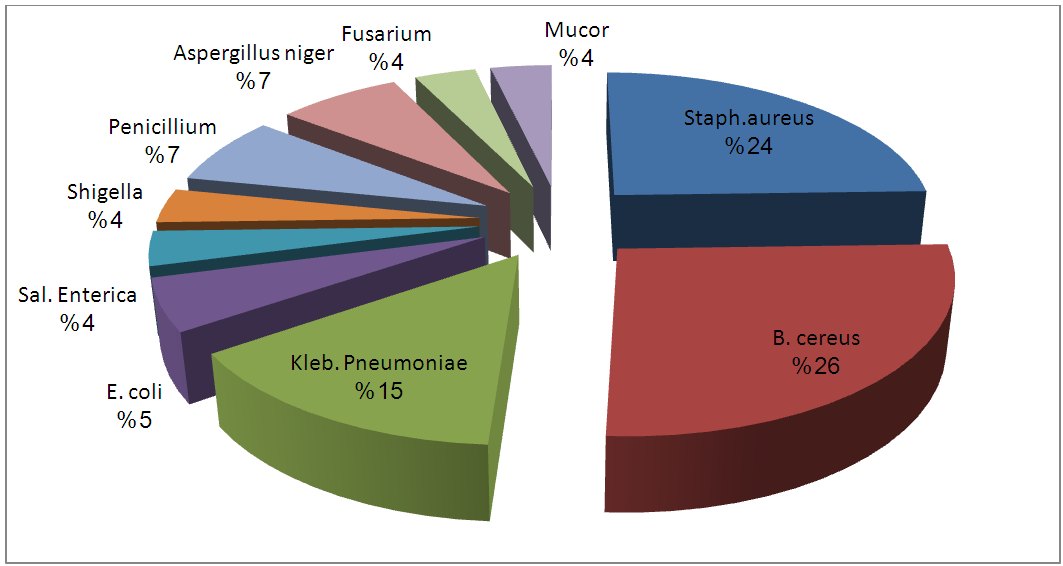 | Figure 6. Percentage Occurrence of Isolates from Unpasteurized Fresh Pineapple Juices Sold in Port-Harcourt |
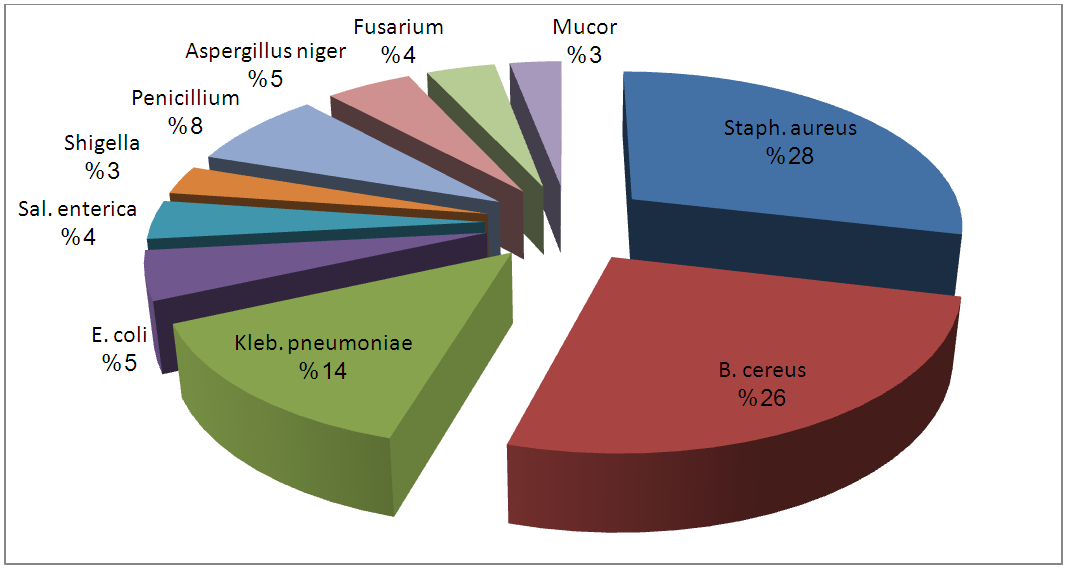 | Figure 7. Percentage Occurrence of Isolates from Unpasteurized Fresh Fruit Juices Sold in Port-Harcourt |
 | Figure 8 |
|
|
5. Discussion
- Different species of micro-organisms may be present in unpasteurized fresh fruit juices during processing, packaging and preservation. The ability of these organisms to survive, multiply and cause spoilage in the juices depends largely on the species of the organism, microbial population and storage conditions. Prolonged storage encourages spoilage of unpasteurized fresh fruit juices irrespective of the nature and origin of the raw material (Beuchat, 1996; Abdussalam and Kafertein, 2012). The total heterotrophic bacteria counts in unpasteurized fresh orange juices from the three locations under study found to be high when assessed using the guidelines for International Commission for Microbiological Specification for Food (≤ 103 cfu/ml). It was observed that 70% of the total samples were over ≤ 103 cfu/ml while 30% were below ≤ 103 cfu/ml. The total heterotrophic count reported in this study is similar with the findings of Cater but lower than the report of Brayant. The presence of heterotrophic organisms in unpasteurized Orange juice sold in Port Harcourt could be attributed to poor handling especially during processing, inappropriate storage conditions and lack of flash pasteurization (Odu and Njoku, 2010). The consumption of such juices could increase the risk of food borne infections such as gastrointestinal infection and salmonellosis caused by pathogenic microorganisms.Also, the total heterotrophic bacteria counts in watermelon juice locally produced in Port Harcourt also exceeded the highest recommended level by ICMSF. Only 10% of the samples were below 103 cfu/ml while the other 90% were above 103 cfu/ml. this result is in accordance with the findings of Doyle (2010). The ability of microorganisms to grow on the juices was largely attributed to less inhibitory nature of watermelon juices due to the higher PH value (5.2), most bacteria survive in alkaline PH better than acidic PH.Pineapple juice also recorded high level of heterotrophic bacteria count. Samples from the three locations were contaminated. Exactly 80% of the samples were over 103 cfu/ml while 20% were below 103 cfu/ml. This ranged from 3.5 x 104 to 6.1x104 cfu/ml. This corresponds with the findings of Sandeep et al. (2002). The relatively high level of mean heterotrophic bacteria counts in unpasteurized Pineapple juice suggests lack of proper hygienic production practices. The coliform counts were higher than the findings of Sneath et al. (2008). The coliform counts were above the ICMSF (1996) which is ≤102 cfu/ml. The presence of coliforms in tested sample suggested poor handling during processing and inappropriate storage conditions. Food borne poisoning associated with the consumption of coliforms contaminated orange juices cannot be over emphasized. This could be largely attributed to raw materials being heavily contaminated with soil (Carmo et al., 2004).Watermelon juices were found to encourage the growth of coliforms because of higher PH value. All the samples from the three locations were contaminated above the recommended standard (Fig. 1). This was largely attributed to poor hygiene, use of contaminated water and poor storage conditions. Coliforms are often associated with poor hygienic production practices and this could increase the risk of food borne illnesses such as travelers’ diarrhea. The level of coliforms reported in this work is in agreement with the findings of Sneath et al. (2008).Pineapple juices from the three locations are contaminated with coliforms and 100% of the samples were contaminated. The level of coliforms reported in this work is a bit higher than the previous report of Sandeep et al. (2002). According to the recommended microbiological standards for any fruit juices sold in the Gulf region the maximum acceptable is 5x104cfu/ml, total coliforms is 100 cfu/ml and yeasts and moulds is 1.0 x 103cfu/ml.The fungi genera isolates of unpasteurized fresh orange juices under study include Penicillium (10%), Fusarium (4%), Mucor sp (2%) and Aspergillus niger (2%). These have been reported by Giberth et al. (2006). Fungal multiplication in these samples could be attributed to poor handling and inappropriate storage conditions. Some of the above mentioned fungi have been confirmed by many researchers to be responsible for the production of mycotoxins which are injurious to health. Watermelon juices from Choba, Mile 3 and GRA were found to be contaminated with similar species of fungi. These species include Penicillium (11%), Fusarium sp (4%), Aspergillus niger (2%) and Mucor sp (2%). The growth of fungi in these samples was attributed to mishandling of these products leading to proliferation of the organisms (Rajav et al., 2004). Pineapple juice locally produced in Port Harcourt were contaminated with different prevalence level of fungi such as Aspergillus niger (7%)and Penicillium sp. (7%), Fusarium sp. (4%) and Mucor (4%); 40 % of the samples under study were above ≤ 102 cfu/ml while 60% were within acceptable standards. The fungal counts reported in this study are in line with the findings of Mensah et al. (2010). Several cases of mycoses have been reported among consumers of unpasteurized pineapple juices contaminated with fungi Rajav et al. (2004). The 4 genera of bacteria isolated from unpasteurized orange juices (Klebsiella pneumoniae, Staphylococcus aureus, Bacillus cereus and E.coli) are in agreement with the report of Odu and Njoku (2010). Abdussalam and Kafertein (2012) suggested that the presence of food borne pathogens in orange juices could be attributed to high water activity, poor handling and cross contamination.Organisms isolated from watermelon juices locally produced in Port-Harcourt include: E. coli, Klebsiella pneumoniae, and Staphylococcus aureus. Some strains of these Enterobacteriaceae have been linked to enterotoxin production and this can pose great health risk (Brayant, 2007). This is because consumption of such juices could lead to food borne infection outbreaks (Odu and Njoku, 2010).The following genera of bacteria were isolated from unpasteurized Pineapple juices: Klebsiella pneumoniae, Salmonella enterica, Staphylococcus aureus, Bacillus cereus and Shigella sp. Organisms mentioned above are commonly linked to poor hygienic production practices with potential food borne infection. These organisms isolated from pineapple juices have been reported by Edema et al. (2004); Odu and Njoku (2010) to be commonly associated with spoilage of fruits and vegetables. Bates et al. (2001) opined that contamination of fruits and juices with pathogenic microorganisms such as E. coli 0157:H7 and Salmonella have caused numerous illnesses and some fatalities. They further stated that all reported cases of contamination by pathogens were due to fresh unpasteurized juices. The profile of pathogens we isolated from tested juices is in agreement with the findings of Bates et al. (2001).
6. Conclusions
- The consumption of unpasteurized fresh fruit juices is difficult to stop due to the health benefits. However, the presence of enteric pathogens such as Escherichia coli and Salmonella enterica in unpasteurized fresh fruit juices would pose a public health risk to their consumers. The result showed that the sanitary quality of the fruit juices were very poor, as a result of poor handling and abuse during processing as reflected by the high microbial load. Hence, it is strongly recommended, that maintenance of good hygiene practices and application of HACCP technique should be adopted to prevent the buildup of spoilage and pathogenic organisms during processing and storage in order to ensure food safety of ready-to-drink (R-T-D) products in south –south, Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
