-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(3): 68-78
doi:10.5923/j.microbiology.20170703.03

Endotoxin Production by Marine E. coli AS10 and Its Antimicrobial Activity
Hassan A. H. Ibrahim 1, Mohamed M. Roushdy 2, Ahmed R. Sofy 2, Ahmed M. Shimy 2, Khalid A. El-Dougdoug 3
1Marine Microbiology Department, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt
2Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
3Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
Correspondence to: Ahmed R. Sofy , Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

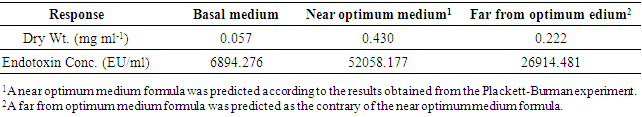
The isolate AS10 was obtained on media for fecal coliforms (mFC agar plates) from Eastern Harbor, Alexandria, Egypt. Phenotypic characteristics using API-20 strips and phylogenetic analysis of 16S rDNA gene nucleotide sequence data confirmed that isolate AS10 was E. coli. The isolate AS10 showed 99.8% sequence homology to E. coli APEC O1 serovar O1:K1 (CP000468). Plackett-Burman design was applied for optimizing the medium components of Endotoxin production. The experimental results demonstrated that the inoculum size (14881.15 EU/ml) was the most effective variable for Endotoxin produced by E. coli AS10, followed by peptone concentration (14076.97 EU/ml). Data also indicated that the main variables which positively affected Endotoxin production concentration were; lactose, bile salt concentration and shaking/static conditions. Yet, sodium chloride and incubation period showed a negative effect. The obtained result was verified and then the optimum medium was predicted. The applied optimum condition resulted in Endotoxin concentration (52058.177 EU/ml, ≈7 fold) increase in the Endotoxin production when compared to the basal medium formulation (6894.276 EU/ml) within the incubation period of 24 h. The optimized medium composition was postulated to maximize Endotoxin production by E. coli AS10 and contained in g/l: peptone, 25; lactose, 15; bile salt, 7.5; sodium chloride, 2.5 and inoculum size, 5 ml under shaking condition within incubation period of 24 h. Using this medium in batch culture gave 52058.177 EU/ml Endotoxin. Antimicrobial activity of Endotoxin produced by E. coli AS10 were evaluated and the results showed its potentiality as potent antibacterial as well as an antifungal agent.
Keywords: Marine E. coli AS10, Plackett-Burman design, Endotoxin production, Antimicrobial activity
Cite this paper: Hassan A. H. Ibrahim , Mohamed M. Roushdy , Ahmed R. Sofy , Ahmed M. Shimy , Khalid A. El-Dougdoug , Endotoxin Production by Marine E. coli AS10 and Its Antimicrobial Activity, Journal of Microbiology Research, Vol. 7 No. 3, 2017, pp. 68-78. doi: 10.5923/j.microbiology.20170703.03.
Article Outline
1. Introduction
- Escherichia coli is the predominant nonpathogenic facultative flora of the human intestine. Most people normally carry harmless strains of E. coli in their intestine. Both the harmless strains and those that cause diarrhea are acquired primarily through ingestion of contaminated food or water. Person-to-person and animal-to-human transmission is through the oral-fecal route. Several strains of E. coli have usually caused diarrhea/gastroenteritis in human which settle within several days without specific treatment [1, 2]. Not all strains of E. coli are pathogenic, but ones that are contribute to one of the leading causes of food borne illnesses [3, 4]. Pathogenic E. coli can be responsible for severe diarrhea in humans and animals and this is produced through the action of toxic proteins known as enterotoxins [5]. However, E. coli is the major facultative anaerobic bacterium in the intestinal tract of most animal species. Typically, in humans it is present in 107-109 organisms per gram of feces. The commensal microbiota is responsible for controlling enteropathogens as many studies have shown production of inhibitory substances by these microorganisms [6]. Toxins of E. coli include; enterotoxins, exotoxins and endotoxins. Endotoxin of E. coli elicits a wide variety of pathophysiological effects. In conditions where the body is exposed to LPS excessively or systemically (as when small concentrations of LPS enter the blood stream), a systemic inflammatory reaction can occur, leading to multiple Pathophysiological effects, such as endotoxin shock, tissue injury, and death [7, 8]. Endotoxin does not act directly against cell or organs, but through activation of the immune system, especially through monocytes and macrophages, with the release of a range of pro inflammatory mediators [9]. On the other side, several clinical applications have been studied, where Michl and Gress [10] reviewed the use of genetically modified bacteria and their toxins targeting advanced solid tumors as a promising new treatment strategy in refractory cancers. Also, Fabbri et al. [11] discussed that the bacterial toxins have increasingly been used as valuable tools for the analysis of cellular physiology, and in the recent years, some of them are used medicinally for the treatment of human diseases. The protein toxins of bacterial origin are currently used in therapy or those under study for their potential clinical applications [12]. Therefore, the current study was suggested to isolate E. coli strains from Egyptian marine sites (Mediterranean Sea and Red Sea). The most promising isolate in the Endotoxin production was fully identified. As well as, the optimization of the Endotoxin production by appropriate experimental design (Plackett-Burman) was employed. Moreover, some potential applications were carried out.
2. Material and Methods
2.1. Isolation of Marine Isolates
- Fifty water samples were collected from subsurface water in Egyptian marine sites, located on the Mediterranean Sea and Red Sea. The samples were held individually .in 500 ml sterile screw-caped glass bottle [13]. Serial dilutions from 10-1 to 10-6 were made using filtered sterilized sea water. A portion (0.1 ml) from each appropriately diluted sample was used to inoculate plates prepared with mFC agar (ISO9308/1, 1992) for isolation of E. coli. Plates were incubated at 44.5°C for 24 h. after incubation; blue colonies were examined in four main characteristics (Gram stain, Cell feature, indole production, and lactose fermentation with gas production) to select colonies of E. coli isolate which will achieve the target of the current study. However, there thirty-three bacterial isolates were visually obtained as E. coli. The isolate E. coli AS10 was chosen as the best growing one.
2.2. Phenotypic Identification of E. coli AS10 Isolate
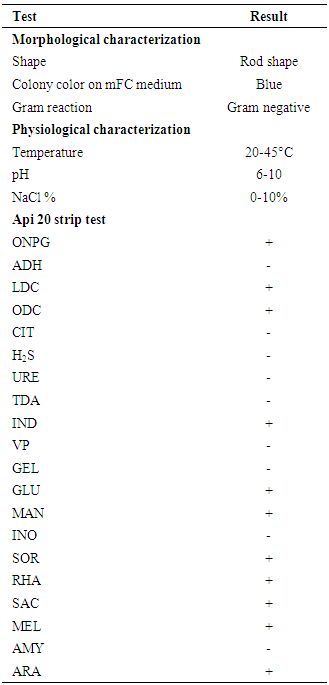
- Phenotypic characteristics such as Gram’s staining, motility, cultural characteristics, catalase, oxidase for the marine bacterial isolate AS10 was studied by adopting standard procedures. Colony morphology characters such as size, appearance, configuration, and elevation were examined on nutrient agar plates after 24 h of incubation period [14]. Effect of sodium chloride, pH level, and temperature on growth was tested. All the tests were performed by adopting standard procedures. For further confirmation, API-20E test for identification and differentiation of members of the family Enterobacteriaceae was applied. The plastic strip holds twenty mini-test chambers containing dehydrated media having chemically-defined compositions for each test.
2.3. Genotypic Identification of E. coli AS10 Isolate
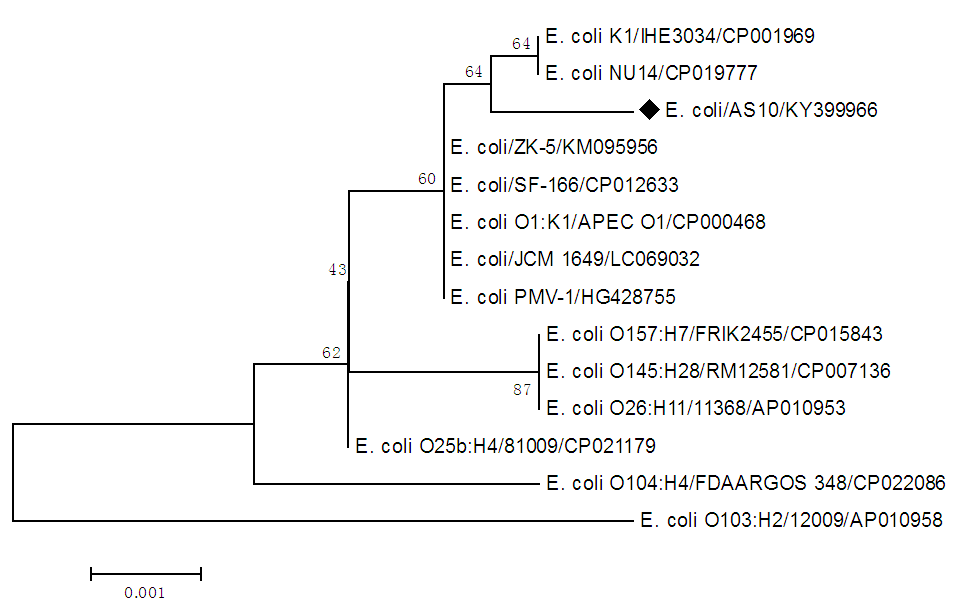
- The genomic DNA of the isolate was extracted using the GFX genomic DNA purification kit (Amersham Bioscience) according to the manufacturer's instruction. The 16S rDNA gene was amplified by PCR using the primers which are: 16S 357 F; ACT CCT ACG GGA GGC AGC AG and 16S 907R; CCG TCAATT CAT TTG AGT TT according to Ausubel et al. [15]. Sequencing was performed using an Applied Biosystems 310 sequencer (ABI 310 DNA sequencer, Big Dye Terminator cycle sequencing ready kit applied biosystems) and the same primers used for PCR. The sequence was compared with similar sequences retrieved from DNA databases by using the NCBI n-BLAST search program at the National Center for Biotechnology Information (NCBI) and aligned with ClustalW (Ver.1.74) program [16]. The nucleotide distances were estimated considering alignment gaps by using Jukes and Cantor [17] method for correction of superimposed substitutions using the Molecular Evolutionary Genetics Analysis (MEGA) software (Ver. 4.0) [18]. Neighbour Joining (NJ) implemented through MEGA 4.0 software and bootstrap analysis (1000 replicates) was performed to assess the reliability of the constructed phylogenetic tree. The 16S rDNA gene nucleotide sequence of the Egyptian isolate marine E. coli (AS10) was registered under GenBank accession number KY399966.
2.4. Optimization of Endotoxin Production by E. coli AS10
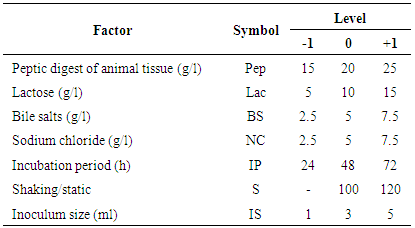
- Plackett-Burman experimental design, a fractional factorial design, was used in this study to demonstrate the importance of some factors implied in Endotoxin production. Seven independent variables were screened in nine combinations organized according to the Plackett-Burman design matrix. For each variable, a high (+1) and low (-1) level was tested [19, 20]. The factors tested were: peptic digest of animal tissue (g/l), lactose (g/l), bile salts (g/l), sodium chloride (g/l), incubation period (h), shaking/static conditions, and inoculum size (ml). The main effect of each variable was determined according to the following equation:
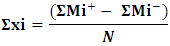 Where:
Where: 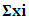 is the variable main effect,Mi+ and Mi- are the response percentage in trials, in which the independent variable xi was present in high and low concentrations, respectively, N is the half number of trials. Nine experiments were generated for the seven factors considered to affect the Endotoxin production. Using Microsoft Excel, statistical t-values of equal unpaired samples were calculated for the determination of variable significance. In order to validate the obtained results and to evaluate the accuracy of the applied Plackett-Burman statistical design, a verification experiment was carried out in triplicates. According to the main effect results, the predicted near optimum and far from optimum levels of the independent variables were examined and compared to the basal condition settings. The Endotoxin concentrations were then estimated as described below.
is the variable main effect,Mi+ and Mi- are the response percentage in trials, in which the independent variable xi was present in high and low concentrations, respectively, N is the half number of trials. Nine experiments were generated for the seven factors considered to affect the Endotoxin production. Using Microsoft Excel, statistical t-values of equal unpaired samples were calculated for the determination of variable significance. In order to validate the obtained results and to evaluate the accuracy of the applied Plackett-Burman statistical design, a verification experiment was carried out in triplicates. According to the main effect results, the predicted near optimum and far from optimum levels of the independent variables were examined and compared to the basal condition settings. The Endotoxin concentrations were then estimated as described below.2.5. Detection and Estimation of Endotoxin from E. coli AS10
- It was carried out by using Limulus Amebocyte Lysate (LAL) PYROGENT-5000 kit, which is a quantitative assay for the detection of Gram negative bacterial Endotoxin. A sample is mixed with the reconstituted LAL reagent, placed in the photometer, and automatically monitored over time for the appearance of turbidity. The time required before the appearance of turbidity (reaction time) is inversely proportional to the amount of Endotoxin present. The LAL kit consisted of two types; N383 and N384 kits with 1 vial of lyophilized Endotoxin per kit. The reconstitution volume of the vial is stated on the Certificate of Analysis, included with each kit, and is calculated to yield a solution containing 100 EU (or IU)/ml. Reconstitute with the specified volume of LAL Reagent Water. Shake vigorously for at least 15 min at high speed on a vortex mixer. Prior to use, a stored stock solution must be warmed to room temperature and vigorously vortexed for 15 min. Store lyophilized Endotoxin at 2-8°C. Reconstituted Endotoxin is stable up to four weeks at 2-8°C. This Endotoxin is provided for the user’s convenience. Other Endotoxin preparations may be used to prepare the standards; however, their performance in the turbidimetric assay relative to the Reference Standard Endotoxin (RSE) must be determined.The incubating microplate reader with the WinKQCL Software was used to run the turbidimetric LAL assay. It was important to become familiar with the operation of the incubating microplate reader and the features of the WinKQCL Software. However, LAL assay was performed in the Delta Pharm Company, Al Obour City, Egypt.
2.6. Extraction of E. coli AS10 Endotoxin
- A phenol-water mixture is employed for the extraction of Endotoxin from E. coli AS10 cells [21]. The bacterial cells washed twice with 4 volumes of 0.85 percent KaCl and once with 4 volumes of sterile distilled water. A supply of such cells was stored frozen at -20°C; washed cells contained 10 to 12 percent of solids. Washed E. coli AS10 cells, 50 g, were suspended in 45 ml of distilled water. The cell suspension was poured into a Waring Blendor containing a mixture of 110 g of phenol and 65 ml of distilled water. The contents of the Waring Blendor were stirred for 8 min. During this period most of the cells disintegrated and the nucleoprotein- Endotoxin complex was hydrolyzed. The temperature of the mixture was 10°C at the beginning, and 42°C at the end of stirring. The mixture was cooled to 20°C, poured into Pyrex centrifuge tubes and centrifuged in an International refrigerated centrifuge at 4°C for 10 min at 3,000 xg. The clear supernatant upper phase was siphoned off and the lower (phenol) phase containing the proteins extracted with 30 ml of distilled water and centrifuged again. A second 50 g portion of bacterial cells was similarly treated (hydrolytic extraction with phenol-water dialysis, and differential centrifugation). The upper phases were combined and centrifuged again for 20 min at 3,000 xg. The combined upper phases were then placed in cellophane bags and dialyzed for 24 h at 4’ against 28 L of distilled water. The latter was stirred continuously and changed occasionally. Phenol phase-from the phenol phase the protein was obtained by the addition of 2.5 volumes of 95 percent ethanol. The precipitate was washed with cold acetone and ether and dried over sodium hydroxide in a vacuum. Aqueous phase contains Endotoxin and nucleic Acid-A small quantity of sodium chloride was added to an aliquot of the dialyzed upper phase and the endotoxin precipitated together with nucleic acid by the addition of 2 volumes of acetone. The precipitate was washed with cold acetone and dried over sodium hydroxide in a vacuum. The yield of dry material was 970 mg per 100 g of wet cells or 9.7 percent on a dry cell basis. This material was water soluble, contained 9.3 percent Kjeldahl-N and 56 percent nucleic acid. The LD50 was 0.8 mg per each 16 to 18 g mouse. Separation of nucleic Acid from Endotoxin by Ultracentrifugation-The major portion of the dialyzed supernatant was centrifuged in a Spinco model L ultracentrifuge for 1 h at 100,000 xg the Endotoxin will be settled in the form of gel-like, transparent material.The sediment was dissolved in 45 ml of distilled water per 100 g of wet cells and centrifuged with angle in a centrifuge at 2,000 xg for 10 min. A slight precipitate was discarded. One hundred mg of sodium chloride (per 100 g of wet cells) were added to the opalescent supernatant and the Endotoxin again precipitated by the addition of 2 volumes of cold acetone. The precipitate was removed by centrifugation, washed with acetone, and dried over sodium hydroxide in a vacuum. The yield of dry Endotoxin was 300 mg per 100 g of wet bacteria or 3 percent on the basis of dry bacteria. The isolated Endotoxin was free from nucleic acid. The LD50 was 0.1 to 0.2 mg. Nucleic Acid-Containing Fraction-A small quantity of sodium chloride was added to the supernatant obtained by ultra-centrifugation (at 100,000 xg) and the nucleic acid was precipitated together with a small quantity of other materials by the addition of 3 volumes of cold acetone. The precipitate was washed with acetone and dried over sodium hydroxide in a vacuum. This material contained 72 to 74 percent nucleic acid and 0.1 to 2.5 percent DNA [22].
2.7. Antimicrobial activity of E. coli AS10 Endotoxin
- Antimicrobial activity of Endotoxin produced by E. coli AS10 was assessed against some pathogenic bacteria (MRSA ATCC 43300, Staphylococcus aureus ATCC 6538, Bacillus cereus ATCC 33018, Klebsiella pneumoniae ATCC 43816, Pseudomonas aeruginosa ATCC 9027, and Salmonella typhi ATCC 14028) and some fungi (Aspergillus niger ATCC 16404, Rhizopus stolonifer ATCC 12938, and Candida albicans ATCC 10231) at concentration 100 micro/ml against bacteria and fungi. All these strains were obtained from the Culture Collection and Identification Unit of the Regional Center for Mycology and Biotechnology, Al-Azhar University, Egypt. The microbial suspension was prepared from a fresh subculture of tested bacteria and fungi in Mueller Hinton Broth (MHB) for bacteria and Sabouraud Dextrose Broth (Difco) for tested fungi, then the suspension was diluted to (106 cfu/ml) using appropriate media. The adjusted microbial inoculums (100µL) were added to each well of sterile 96-well flat-bottomed microtiter plate containing the tested concentration 100 (micr/ml) of endotoxin 100 (µL/well). As a result, last inoculum concentration of (5x105 CFU/ml) was obtained in each well. Three wells containing microbial suspension without tested extract used as (Growth control) and two wells containing only media as (background control) were included in this plate. Optical densities were measured at (620 nm) after 24h at 37°C for bacteria and 48 h at 28°C for fungi using an ELISA microplate reader (Sunrise™-TECAN, Switzerland) at the Botany and Microbiology Department Faculty of science Al-Azhar University. Finally, cell concentrations were transformed to a percentage of bacterial and fungal inhibition. The percentage of growth reduction (GR %) was estimated using as reference the control treatment (without endotoxin) as
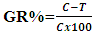 Where, C is the cell concentrations under the control treatment and T is the cell concentrations under the treatment. Three replicates were considered. The results were recorded as means ±SE of the triplicate experiment. MIC also determined using the same procedure using two-fold serial dilution at concentrations ranged from 100, 50, 25, 12.5, 6.25, 3.125, 1.562 and 0.781 micr. and the 96-well flat-bottomed microtiter plate measured at (620 nm) after 24 h at 37°C for bacteria and after 48 h at 28°C for fungi using ELISA microplate reader [23].
Where, C is the cell concentrations under the control treatment and T is the cell concentrations under the treatment. Three replicates were considered. The results were recorded as means ±SE of the triplicate experiment. MIC also determined using the same procedure using two-fold serial dilution at concentrations ranged from 100, 50, 25, 12.5, 6.25, 3.125, 1.562 and 0.781 micr. and the 96-well flat-bottomed microtiter plate measured at (620 nm) after 24 h at 37°C for bacteria and after 48 h at 28°C for fungi using ELISA microplate reader [23].3. Results
3.1. Phenotypic Identification of Marine Isolate AS10
- The isolate AS10 was obtained on mFC agar plates from Eastern Harbor. Scanning electron microscopic of bacterial isolate AS10 shown as rods. Moreover, table (1) shows some phenotypic characteristics of the selected isolate AS10. These included colony and cell morphology, Gram reaction, indole production, gas production, catalase, and oxidase test in addition to some physiological and biochemical experiments. The results showed that it is a Gram-negative rod bacterium. It grew at 20-45°C, pH range (6-10) and at all examined sodium chloride tested concentrations (0-10%). In addition, several tests were examined. Some of them showed positive and others recorded produced negative results.
|
3.2. Genotypic Identification
- PCR amplification of the 16S rDNA gene produced the expected amplicon size of approximately 1500 bp. The partial nucleotide sequence of isolate AS10 16S rDNA gene (1166 nucleotides) was compared with similar sequences retrieved from DNA databases by using the NCBI n-BLAST search program at the National Center for Biotechnology Information (NCBI). It was multiple-aligned at the same partial sequences of 13 reported E. coli sequences in GenBank using the ClustalW program with minor manual adjustments, where the number of base substitution per site from between sequences is shown in table (2). Standard error estimate (s) are shown above the diagonal.
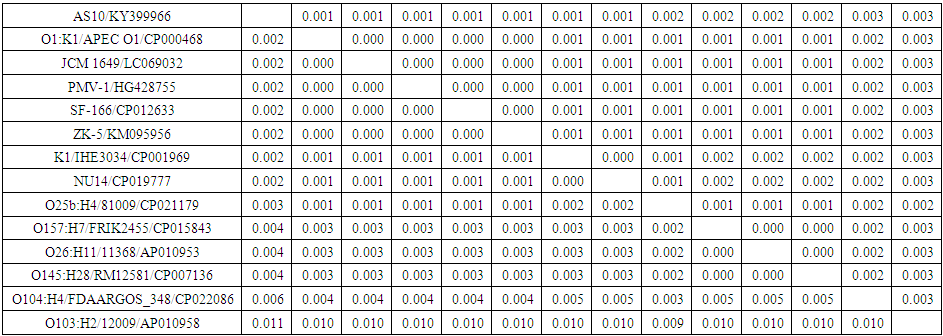 | Table 2. Pairwise distances of nucleotide sequences of partial 16S rDNA gene of E. coli isolate AS10 (accession no. KY399966) and 13 E. coli sequences published in GenBank |
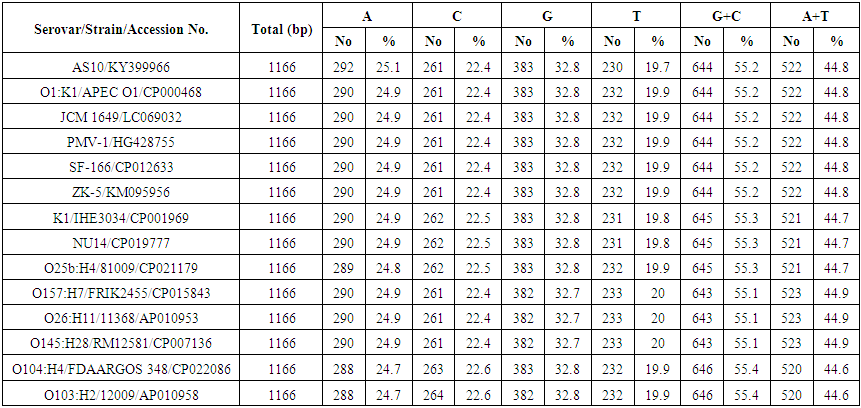 | Table 3. Comparison between base composition of 16S rDNA gene of E. coli isolate AS10 (accession no. KY399966) and 13 E. coli sequences published in GenBank |
3.3. Optimization of Endotoxin Production by E. coli AS10
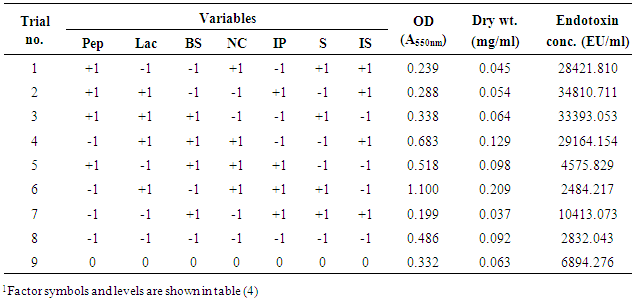
- In this experiment, Plackett-Burman design was applied to evaluate the effective factors and study their interaction and select optimum conditions in a limited number of experiments. The chosen levels of the culture components are given in tables (4 & 5). The design was applied with 7 different variables and hence 8 different growth conditions are presented in table (5). The experimental results demonstrated that trial no. 2 supported the highest Endotoxin production (34810.711 EU/ml) by E. coli AS10, while trail no. 6 supported the highest dry weight (0.209 mg/ml) production by E. coli AS10.
|
|
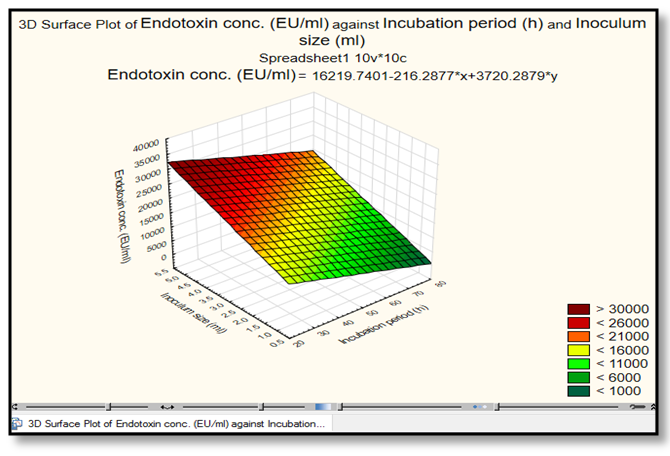
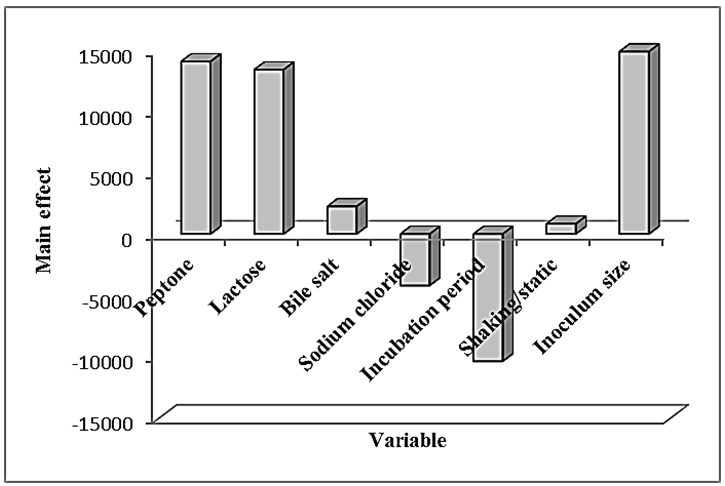 | Figure 2. Elucidation of the cultured factors that affect the production of Endotoxin by E. coli AS10 |
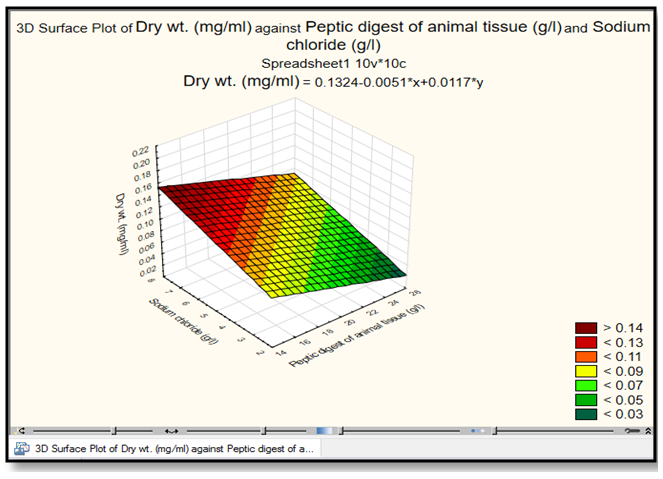
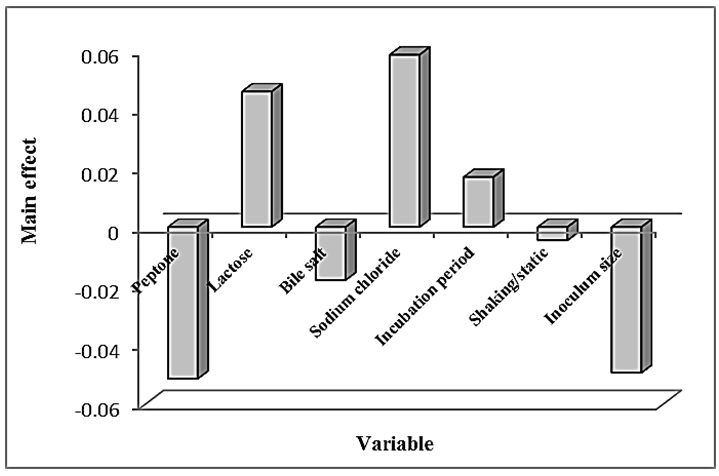 | Figure 3. Elucidation of the cultured factors that affect dry weight of E. coli AS10 |
|
3.4. Antimicrobial Activity of Endotoxin
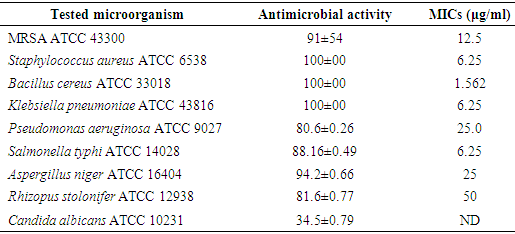
- The antibacterial/antifungal activities of the Endotoxin against microorganisms showed that the antibacterial activity ranged between 80.6-100%. The highest inhibition percentages were detected against S. aureus ATCC 6538, B. cereus ATCC 33018 and K. pneumoniae ATCC 43816, while the lowest % was found against P. aeruginosa ATCC 9027. On the other hand, the MICs against the previous microorganisms were concentration dependent. S. aureus ATCC 6538, K. pneumoniae ATCC 43816 and S. typhi ATCC 14028 were inhibited at the same concentration recording MIC value 6.25 μg/ml. While, the MRSA ATCC 43300 and P. aeruginosa ATCC 9027 required more endotoxin concentrations to be inhibit recording the highest MIC values at 12.5 μg/ml and 25 μg/ml, respectively (Table 7).
|
4. Discussion
- Bacterial indicators are used by many investigators to assess the effluent discharged into coastal/marine environments and distribution of pathogenic bacteria [24]. Among of them, E. coli was detected in many sites of the Red Sea and Mediterranean Sea, Egypt [25, 26].Escherichia coli is Gram-negative, facultative anaerobic and non-sporulating. It can live on a wide variety of substrates. Escherichia coli use mixed-acid fermentation in anaerobic conditions, producing lactate, succinate, ethanol, acetate and carbon dioxide. Since many pathways in mixed-acid fermentation produce hydrogen gas, these pathways require the levels of hydrogen to be low, as is the case when E. coli lives together with the hydrogen-consuming organisms such as methanogens or sulfate-reducing bacteria [27]. Optimal growth of E. coli occurs at 37°C, but some laboratory strains can multiply at temperatures of up to 49°C [4, 28, 29, 30].Endotoxins (Lipopolysaccharides) are the main component of the cell membrane of almost all gram negative bacteria. It is responsible for the pathological consequences of gram negative bacterial infections [31, 32].The isolate AS10 was obtained from the Eastern Harbor on mFC agar plates. Phenotypic characteristics of the selected isolate AS10 using API-20 strips.Bueschkens and Stiles [33] confirmed the fecal E. coli characteristics of gas production from lactose and indole production at elevated incubation temperatures. Endotoxin of E. coli AS10 was estimated by LAL assay using microplate reader and factors affecting its production were determined. Endotoxins are an important structural component of the outer cell membrane complex of gram negative microorganisms. Its causative role in gram negative bacteria-induced diseases and broad applications in different kinds of cell stimulation experiments provided a conceptual basis for studies directed at the isolation, purification, and detailed chemical characterization of Endotoxin.All Endotoxin samples in the present study were measured using LAL assay, which is considered a successful assay that was developed to measure Endotoxin concentration based on the observations of Fred Bang, a Marine Biological Laboratory scientist, that Gram-negative bacteria, even if killed, will cause the blood of the horseshoe crab (Limulus Polyphemus) to turn into a semi-sold mass [31, 34].Currently, there are three forms of the LAL assay, each with different sensitivities. The LAL gel clot assay can detect down to 0.03 EU/ml, while the LAL kinetic turbidimetric and chromogenic assays can detect down to 0.01 EU/ml [34].Mamat et al. [35] reviewed that the Endotoxin is a complex lipopolysaccharide (LPS) found in the outer cell membrane of gram-negative bacteria. Endotoxins consist of a core polysaccharide chain, O-specific polysaccharide side chains (O-antigen) and a lipid component, Lipid A, which is responsible for the toxic effects. Endotoxins are approximately 10 kDa in size, but readily form large aggregates up to 1000 kDa. Bacteria shed endotoxin in large amounts upon cell death and when they are actively growing and dividing. A single E. coli contains about 2 million LPS molecules per cell. Ryan [31] mentioned that the Endotoxins have a high heat stability, making it impossible to destroy them under regular sterilizing conditions. They are amphipathic molecules that carry a net negative charge in solution. Because of their hydrophobicity, they are likely to have strong affinities for other hydrophobic materials like plastic products used in the laboratory. For this reason, carryover contamination from laboratory beakers, stir bars, and other lab ware is common.Optimization of Endotoxin production by E. coli AS10 was carried out using Plackett-Burman design. Plackett-Burman design offers good and fast screening procedure and mathematically computes the significance of large number of factors in one experiment which is time saving and maintains convincing information on each component. This indicates the effectiveness of this design as a tool for elucidating the most important variables affecting the response.The results pointed out that the peptone, lactose and inoculum size were the most significant variables on the Endotoxin produced by E. coli AS10. The optimization experiments formulated an optimized production medium for E. coli AS10 Endotoxin production containing the following components in g/l: peptone, 25; lactose, 15; bile salt, 7.5; sodium chloride, 2.5 and inoculum size, 5 ml under shaking condition within incubation period of 24 h. Using this medium in batch culture gave 52058.177 EU/ml Endotoxin.Rawia et al. [36] obtained a high cell biomass (8.8 g/l) and Endotoxin concentration (6.8 mg/l), by the shake flask culture (100 ml medium 250 ml flask, at 200 rpm), of the recombinant strain of Escherichia coli in modified MR medium containing sucrose (20 g/l), as carbon source and yeast extract as a nitrogen source, in the presence of CaCO3, K2HPO4, MgSO4, FeSO4 and ZnSO4 as mineral salts. The best pH values for cell biomass production and endotoxin production were 7.0 and 7.5, respectively. The corresponding figures for the best temperature were 37 and 30°C, respectively. Investigations on E. coli Endotoxin are rather less than as they should. Most of them have been studied the isolation and confirmation of E. coli as fecal indicator bacteria, the removal of its Endotoxins, optimizing the growth of its recombinant strains or the antibacterial and anti-inflammatory agents that target its Endotoxin. So, the present work is considered the first study that deals with the optimization of its Endotoxins production and its antimicrobial action against pathogenic bacteria and fungi.The Endotoxin of E. coli AS10 exhibited strong antibacterial and antifungal activities against all the tested microorganisms (in exception C. albicans ATCC 10231). In addition, the MIC values were concentration dependent against all the tested microorganisms, while not detected against C. albicans ATCC 10231.Schmitt et al. [37] reviewed the role certain toxins have played in unraveling signal pathways in eukaryotic cells and summarize the beneficial uses of toxins and toxoids. Our intent is to illustrate the importance of the analysis of bacterial toxins to both basic and applied sciences.For a long time, bacterial toxins were considered mainly as the pathogenicity factors or potential components of bacteriological weapons. The situation has been gradually changed, since some toxin properties can be more useful rather than harmful. Thus, attempts to use the botulinum toxin for the eye muscle relaxation were made in the 70s-80s [38]. These experiments initiated multimillion markets: now the botulinum toxin is used in neurology for treatment of different neuromuscular diseases and in cosmetology to get rid of wrinkles and decrease diaphoresis [39, 40].
5. Conclusion
- The present work has identified phenotypically and genotypically the most promising E. coli strain, which was isolated from Egyptian marine sites, in the Endotoxin production. As well as, the optimization of the Endotoxin production by Plackett-Burman design was employed. The data predicted the optimized medium composition to maximize the Endotoxin production and contained in g/l: peptone, 25; lactose, 15; bile salt, 7.5; sodium chloride, 2.5 and inoculum size, 5 ml under shaking condition within incubation period of 24 h. The optimum condition resulted in Endotoxin concentration (≈7 fold) increase in the Endotoxin production when compared to the basal medium formulation. Furthermore, the potential antimicrobial activity was detected against pathogenic bacteria and fungi.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML