-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(3): 55-67
doi:10.5923/j.microbiology.20170703.02

Effectiveness of Chitosan as Naturally-Derived Antimicrobial to Fulfill the Needs of Today’s Consumers Looking for Food without Hazards of Chemical Preservatives
Ahmed A. Hmed1, Ahmed R. Sofy1, Abd El-Monem M. A. Sharaf1, Khalid A. El-Dougdoug2
1Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt
2Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
Correspondence to: Ahmed R. Sofy, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

With consumers growing awareness and concerns regarding chemically synthesized preservatives, novel and safe natural antimicrobials targeting food pathogens with minimum adversative effects have attracted much more attention. So, the overall objective of this study is to develop natural antimicrobials (chitosan), for controlling the growth of foodborne pathogens and spoilage microorganisms with good potential for use as preservative agents to improve food safety. Evaluation of the antimicrobial efficacy of chitosan was assessed against five foodborne bacterial isolates, including Bacillus cereus, Staphylococcus aureus, Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa. As well as, against a panel of fungi/yeast including Aspergillus niger, Aspergillus flavus, Penicillium italicum, Rhizopus stolonifer and Candida albicans. The minimum inhibitory concentration (MIC) using a broth microdilution method against tested microorganisms and the biofilm inhibition activity against foodborne bacterial isolates were performed. The chitosan possessed strong antibacterial/antifungal activities against all tested microorganisms with markedly stronger antifungal activity. Moreover, Gram-negative bacteria more affected by chitosan than Gram-positive one. Based on their MIC values, chitosan showed a broad spectrum of actions against tested Gram-positive, Gram-negative bacteria and fungi/yeast strains, and this effect was concentration dependent. As well as, chitosan was able to overcome the biofilm formation of some tested bacteria as dose-dependent manner, at sub-MICs. Finally, the effectiveness of chitosan against foodborne pathogens and spoilage microorganisms demonstrated in this study may prompt future applications of chitosan as a promising alternative of traditional antimicrobials in food biopreservation to fulfill the concept of “natural” or “healthy” foods that consumers are increasingly demanding.
Keywords: Chitosan, Biopreservation, Foodborne bacteria, Antibacterial activity, Antifungal activity, MIC, Antibiofilm activity, Healthy food
Cite this paper: Ahmed A. Hmed, Ahmed R. Sofy, Abd El-Monem M. A. Sharaf, Khalid A. El-Dougdoug, Effectiveness of Chitosan as Naturally-Derived Antimicrobial to Fulfill the Needs of Today’s Consumers Looking for Food without Hazards of Chemical Preservatives, Journal of Microbiology Research, Vol. 7 No. 3, 2017, pp. 55-67. doi: 10.5923/j.microbiology.20170703.02.
Article Outline
1. Introduction
- Traditional antimicrobials have been used as reliable preservatives to control microbial hazards in the food industry for decades [1]. Although these compounds, synthetic and semisynthetic, have been widely accepted in the modern era, the undesirable side effects cannot be neglected and do not satisfy the concept of “natural” or “healthy” foods that consumers are increasingly demanding. So, there is a need for new, more efficient antimicrobials for use in food products to ensure that consumers have access to a safe food supply. Due to the negative impact from chemical preservatives, attention has shifted to the use of naturally-derived antimicrobial agents to control foodborne pathogens. With the increasing claim for food safety and health standards, consumers have become more concerned about the occurrence of chemical residues in the food products [2]. On the other hand, natural antimicrobials can be used as a promising alternative of traditional antimicrobials for preserving foods [1]. Natural antimicrobials are derived from many sources, including animals (chitosan) [2].Chitosan has attracted much commercial interest with regards to medical, pharmaceutical, and industrial applications due to the possession of several interesting properties: biodegradability, biocompatibility, and low toxicity [3]. Additionally, other properties such as analgesic, antitumor, hemostatic, hypocholesterolemic, antimicrobial, and antioxidant properties of chitosan have also been reported [4]. The antimicrobial activity of chitosans has been observed in a wide variety of microorganisms, including bacteria, yeast, and fungi [5]. Hence, the aim of this study was to determine the antibacterial/antifungal activity of chitosan against representative foodborne Gram-positive and Gram-negative pathogens and spoilage microorganisms. Also, to evaluate the MIC values generated by broth microdilution and determine the antibiofilm activity against foodborne bacterial isolates biofilm.
2. Material and Methods
2.1. Chitosan Preparation
- Chitosan compound (MW= 67 kDa; DD= 80) prepared from crab shell waste was obtained from Zoology Department, Faculty of Science (Boys), Al-Azhar University, Cairo, Egypt. Chitosan was dissolved in acetic acid (0.1%, v/v) to obtain a stock solution (10%, w/v). The pH value of the solution was adjusted to 5.9 with 1 N HCl and 1 N NaOH. The stock solution was filter sterilized through a 0.2 μm filter (BD Diagnostic Systems) and kept in the refrigerator before use [6].
2.2. Tested Microorganisms
2.2.1. Bacteria
- Five food isolates were Bacillus cereus, Staphylococcus aureus, Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa, identified according to Bergey's manual [7]. API and Biolog system were performed for confirming the identification.
2.2.2. Fungi/Yeast
- Five food isolates were Aspergillus niger, Aspergillus flavus, Penicillium italicum, Rhizopus stolonifer and Candida albicans obtained from Mycology Laboratory of the Department of Botany and Microbiology, Faculty of Science (Boys), Al-Azhar University, Cairo, Egypt.
2.3. Antimicrobial Susceptibility Testing
2.3.1. Disk Diffusion Assay
- Impregnated paper discs (6 mm in diameter; BD Diagnostic Systems) with chitosan in concentration 5% were placed on the surface of inoculated each MHA plate using a sterile pair of forceps (~4 mm thickness agar layer). The Petri dishes were sealed using parafilm and left 1 hr in the refrigerator. Negative controls were done using sterile distilled water instead of chitosan. Then, plates were incubated at 37°C for 24 hr. The susceptibility of the bacteria to the chitosan was estimated by measuring the diameter of inhibition zones using a ruler or caliper and recorded values as the average of three replicates [8].
2.3.2. Agar Well Diffusion Assay
- Sabouraud Dextrose Agar (SDA), (Difco) was inoculated with tested organisms before the agar solidification. Discs of the inoculated agar were cut with Cork borer and removed to make agar wells, where tested chitosan in concentration 5% filled these wells. Controls Petri plates were prepared using distilled water instead of chitosan. Replicates of each treatment were incubated at 28°C for 2-4 days. The susceptibilities of fungi/yeast to chitosan were estimated by measuring the diameter of the zones of inhibition. The results were recorded as the average of three replicates [9].
2.3.3. Broth Microdilution Assay
- Suspension equivalent to the turbidity of 0.5 McFarland standard (108 cfu/ml) prepared from a fresh subculture in Mueller Hinton Broth (MHB), then the suspension was diluted to 106 cfu/ml using MHB for bacteria and SDB for fungi/yeasts. The adjusted microbial inoculums (100 µl) were added to each well of sterile 96-well flat-bottomed microtiter plate containing the tested concentration of chitosan (100 µl/well). As a result, last inoculum concentration of 5x105 cfu/ml was obtained in each well. Three wells contain a microbial suspension without chitosan used as growth control and two wells containing only media as a background control were included in the plate. Optical densities were measured at 620 nm after 24 hr at 37°C for bacteria and 48 hr at 28°C for fungi/yeast using an ELISA microplate reader (Sunrise™-TECAN, Switzerland) at the Botany and Microbiology Department, Faculty of Science, Al-Azhar University. Finally, cell concentrations were transformed to a mean growth inhibition percentage (%). The percentage of microbial growth reduction (GR %) was estimated using as reference the control treatment (without extract) as:
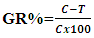 Whereby C is the cell concentrations under the control treatment, and T is the cell concentrations under the extract treatment. Three replicates were considered. The results were recorded as means ±SE of the triplicate experiment [10].
Whereby C is the cell concentrations under the control treatment, and T is the cell concentrations under the extract treatment. Three replicates were considered. The results were recorded as means ±SE of the triplicate experiment [10].2.3.4. Determination of Minimal Inhibitory Concentration (MIC)
- The MIC was determined by Broth Microdilution Method. Two-fold serial dilution in the same type of broth media of tested chitosan was diluted to yield concentrations 5-0.07%, and sterile distilled water was added to the wells of the negative control. The microtitre plates were prepared by adding 100 μl of the same type of appropriate broth media for bacteria and fungi/yeast. Serial dilution was carried out up to well number 12 from which 100 μl were discarded. Twenty microliters of the microbial suspension were added to each well, except the control wells. An automatic ELISA microplate reader (Sunrise™-TECAN, Switzerland) adjusted at 600 nm was used to measure the absorbance of the plates before and after incubation at 37°C after 24 hr for bacteria and after 48 hr at 28°C for fungi/yeast. The absorbencies were compared to detect an increase or decrease in the growth, and the values plotted against concentration. The lowest concentration of chitosan resulting in inhibition of bacterial or fungal growth was recorded as the MIC [10].
2.4. Antivirulence Activity
2.4.1. Biofilm Formation Assay of Bacterial Isolates
- Tissue culture plate method was used as a quantitative test for detection of biofilm described by Christensen et al. [11]. Bacteria isolated from fresh agar plates were inoculated with 10 ml of trypticase soy broth with 2% glucose. The inoculated broths were incubated at 37°C for 24 hr. The cultures were then diluted (1:100) with fresh medium. Individual wells of sterile 96-well, flat bottom polystyrene tissue culture treated plates (Sigma-Aldrich, Costar, USA) were filled with 200 μl of the diluted cultures. Negative control wells inoculated with 200 μl sterile broth medium. The plates were incubated at 37°C for 24 hr. After incubation, the contents of each well were removed (free floating bacteria) by gentle tapping. The wells were washed with 0.2 ml of phosphate buffer saline (pH=7.2) four times. Biofilm formed by bacteria adherent to the wells were fixed by 2% sodium acetate and stained with crystal violet (0.1%). Excess stain was removed by using deionized water and plates were kept for drying. Optical density (O.D.) of stained adherent biofilm was obtained by using an ELISA microplate reader (Sunrise™-TECAN, Switzerland) at wavelength 570 nm. The experiment was performed in triplicate and repeated three times. The interpretation of biofilm production was done according to the criteria of [12].
2.4.2. Biofilm Inhibition Assay
- Chitosan was tested for its potential to prevent biofilm formation of bacterial isolates at sub (MICs) against each bacterium. An aliquot of two-fold serial dilutions was prepared in the 96-well microtiter plate containing trypticase soy broth with 2% glucose (TSBGlc) to obtain the previous concentrations in 100 μl. Bacterial suspensions (50 μl; 5×105 cfu/ml, final concentration) were then transferred into the plate. TSBGlc containing distilled water was employed as a negative control. Inoculated TSBGlc without chitosan was used as the positive control. Following incubation at 37°C for 24 h, the effect of chitosan on the bacterial growth was evaluated using the microplate reader at an optical density of 570 nm. The bacterial biofilm formation in the presence of the tested extract was subsequently determined and compared with the positive control [13].
2.5. Time Kill Assays
- Tested chitosan (1.25% for S. aureus and 3.12% for P. aeruginosa) was diluted with 0.1% acetic acid. Suspensions were mixed with bacteria harvested at the late logarithmic phase and diluted to approximately 4.0-5.0 log cfu/ml. A total volume of 25 ml was used consisting of 12.5 ml of trypticase soy broth (TSB), 10 ml of filtered dialyzed extract and 2.5 ml of inoculum. Then, incubated at 37°C for the interval period of times (0, 3, 6, 12 and 24 hrs). A bacterial suspension (1 ml) collected and the effect of the tested chitosan on the bacterial growth was evaluated at each previous times using the spectrophotometric assay at an optical density of 620 nm. Also, 1 ml of bacterial suspension was plated in triplicate using tryptic soy agar, incubated for 24 hrs, at 37°C, and then cfu enumerated [14].
2.6. Preparation of Samples for Scanning Electron Microscopy
- Treated and non-treated samples were fixated by glutaraldehyde (2.5%) and dehydrated by serial dilution of ethanol using an automatic tissue processor (Leica EM TP). Then, the samples were dried using CO2 critical point drier (Tousimis Audosamdri-815). The samples were coated by gold sputter coater (SPIModule) and examined by scanning electron microscopy (JEOL-JSM-5500LV) by using high vacuum mode at the Regional Center of Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt.
3. Results
3.1. Antibacterial Activity
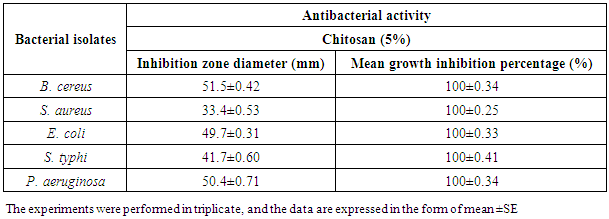
- Chitosan exhibited a wide-range of bioactivity as an antibacterial against Gram-positive and Gram-negative tested bacterial isolates. The results illustrated in Fig. (1) and presented in table (1) showed the inhibition zone diameters against B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa were 51.5±0.42, 33.4±0.53, 49.7±0.31, 41.7±0.60 and 51.5±0.42 (mm), respectively, and the mean growth inhibition percentage was 100% against all tested bacteria showing a broad spectrum of activity and higher killing rate against all tested bacteria.
|
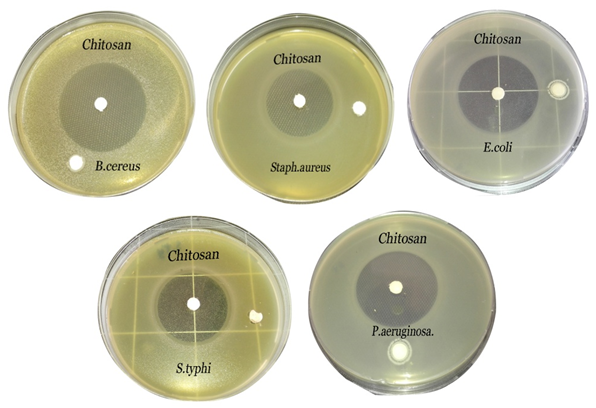 | Figure 1. Inhibition zones produced against B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa using chitosan (5%) |
3.2. Antifungal Activity
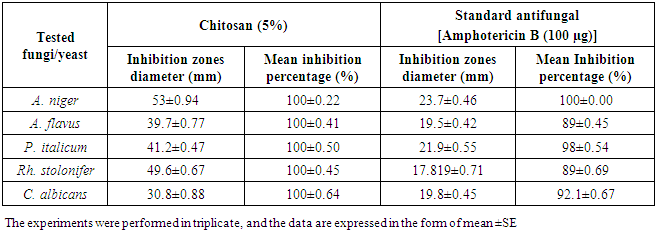
- Chitosan showed strong antifungal activity against all tested strains, which were more susceptible depending by inhibition zone (hallo) diameters and mean growth inhibition percentages (Fig. 2 & Table 2). The inhibition zone diameters of chitosan against A. niger, A. flavus, P. italicum, Rh. Stolonifer and C. albicans, were 53±0.94, 39.7±0.77, 41.2±0.47, 49.6±0.67and 30.8±0.88 (mm), respectively, and the mean growth inhibition percentages were 100% against all tested fungi/yeast (Fig. 2 & Table 2).
|
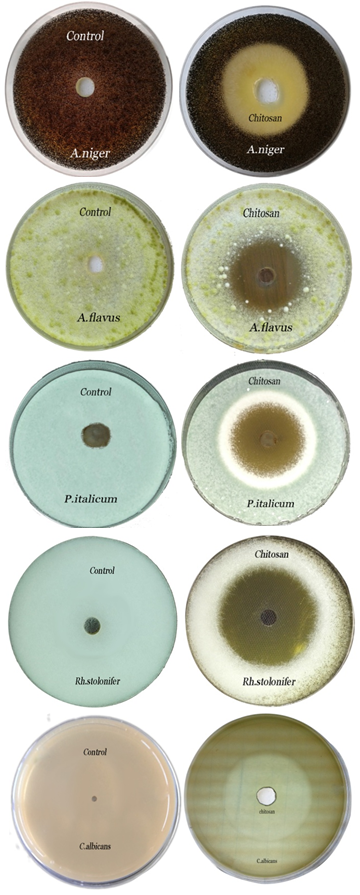 | Figure 2. Inhibition zones produced against A. niger, A. flavus, P. italicum, Rh. stolonifer and C. albicans using chitosan (5%) |
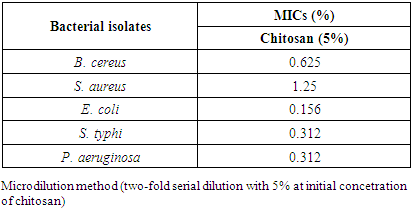
3.3. MICs against Tested Bacterial Isolates
- The reported MICs of chitosan were varying widely with the bacterial wall type, ranging from 0.312% to 1.25% (Table 3). Chitosan has been shown to inhibit both Gram-positive and Gram-negative bacteria, including B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa, but Gram-negative bacteria appeared to be more sensitive. The results obtained from the table (3) and Figs. (3 & 4) showed that, a much higher concentration of chitosan is required for complete inhibition of Gram-positive bacteria especially S. aureus than Gram-negative one. The MICs values were 0.625, 1.25, 0.156, 0.312 and 0.312% against the previous tested bacteria, respectively (Table 3).
|
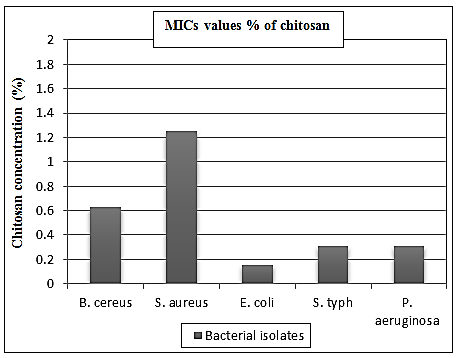 | Figure 4. MICs values of chitosan against different bacterial isolates |
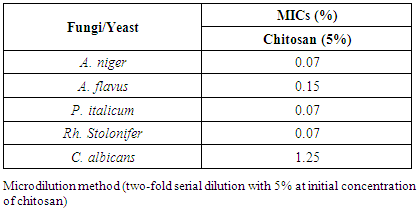
3.4. MICs against Tested Fungi/Yeast
- According to the results shown in Fig. (5) and designated in the table (4), chitosan showed strong antifungal activity with low MICs values towards A. niger, A. flavus, P. italicum, Rh. stolonifer and C. albicans. The MICs values recorded 0.07% against A. niger, P. italicum, and Rh. Stolonifer, while at concentrations 0.15% and 1.25%, A. flavus and C. albicans were inhibited. C. albicans showed the highest MIC value among the tested organisms (Table 4 and Fig. 6).
|
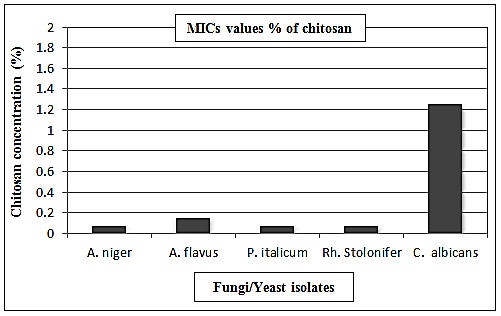 | Figure 6. MICs values of chitosan against fungi/yeast isolates |
3.5. Antivirulence Activity
3.5.1. Biofilm Inhibition Activity
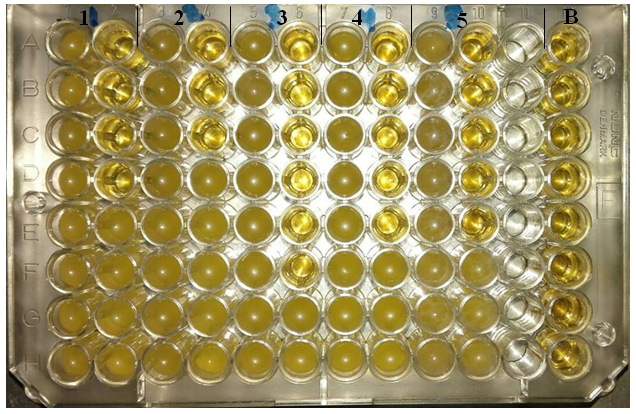
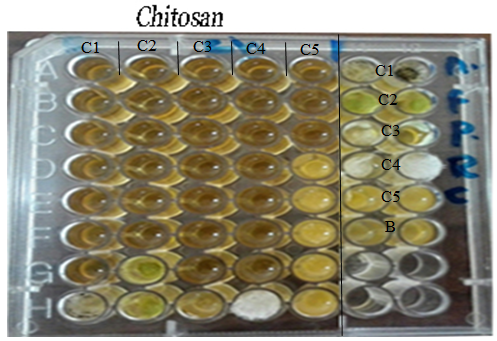
- Doses of sub-inhibitory concentrations from chitosan showed different effects ranging from weak, moderate and strong influence on bacterial biofilms against B. cereus, S.. aureus, E. coli, S. typhi and P. aeruginosa (Fig. 7).
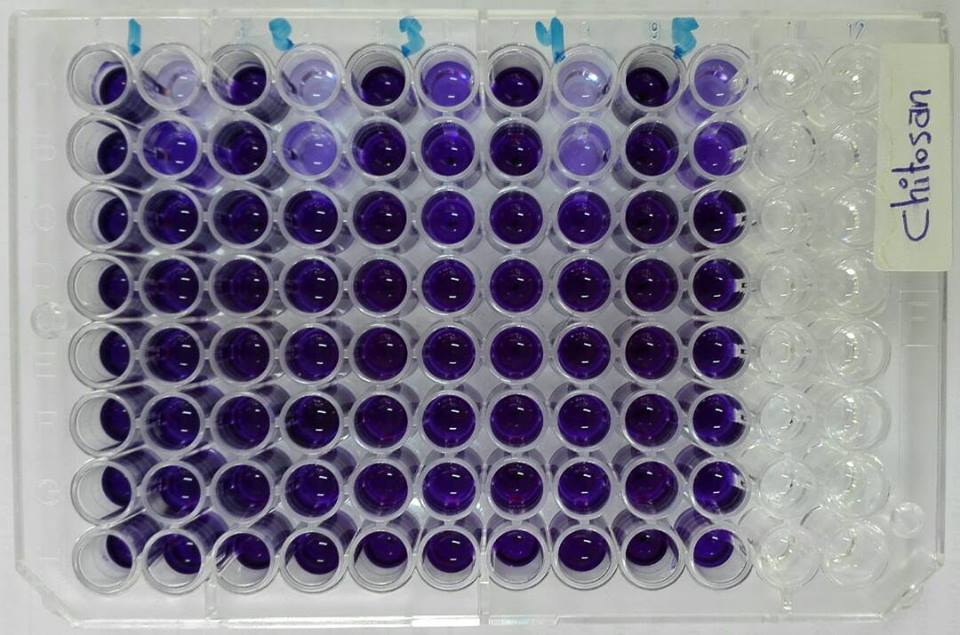 | Figure 7. Microtiter ELISA plate is showing biofilm inhibition assay of chitosan against bacterial isolates. 1) B. cereus, 2) S. aureus, 3) E. coli, 4) S. typhi and 5) P. aeruginosa |
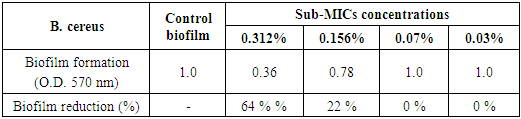
3.5.1.1. Bacillus cereus Biofilm
- The results illustrated in Figs. (7 & 8) and presented in table (5) showed the antibiofilm efficacy of chitosan at sub-MIC against B. cereus biofilm. Since, chitosan showed a reduction activity represented 64% and 22% at [½ MIC=0.312% and ¼ MIC=0.156%], while at [1/8 MIC=0.07%] showed no biofilm reduction activity, whereby at this concentration the biofilm resembles the control positive one.
|
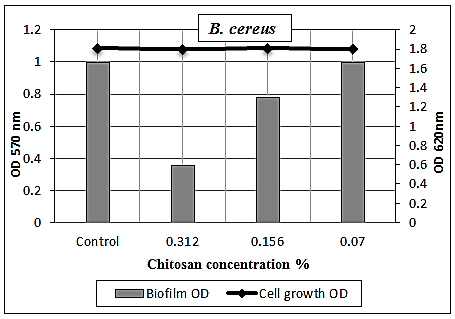 | Figure 8. Sub-MICs of chitosan against B. cereus biofilm. Bacterial growth is linear chart, and the biofilm formation is column charts. The control is 0% chitosan concentraion |
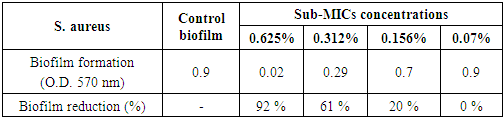
3.5.1.2. Staphylococcus aureus Biofilm
- The chitosan was successfully able to smash up S. aureus biofilm especially at [½ MIC=0.625%] as dose dependent manner with strong reduction percentage 92% and with moderate eradication effect 61% and 20% at concentrations [1/4 MIC=0.312%, 1/8 MIC=0.156%], respectively (Table 6 and Figs. 7 & 9). Also, it was found that the (O.D.570) of [1/16 MIC=0.07 %] equal the (O.D.570) of positive control biofilm of the pathogen without treatment, where at this concentration the antibiofilm activity of chitosan became effectiveless.
|
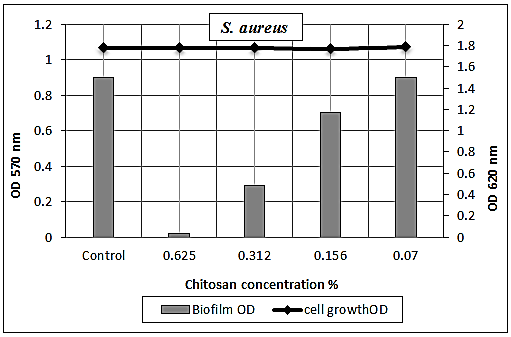 | Figure 9. Sub-MICs of chitosan against S. aureus biofilm. Bacterial growth is linear chart, and the biofilm formation is column charts. The control is 0% chitosan concentraion |
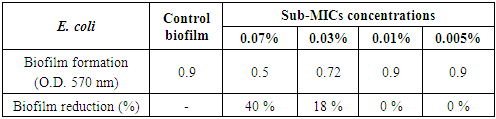
3.5.1.3. Escherichia coli Biofilm
- The concentration-dependent analysis confirms that chitosan at sub-inhibitory concentrations (0.07 and 0.03%) had a moderate effect against E. coli biofilm with eradication percentages (40% and 18%, respectively) (Table 7 and Figs. 7 & 10).
|
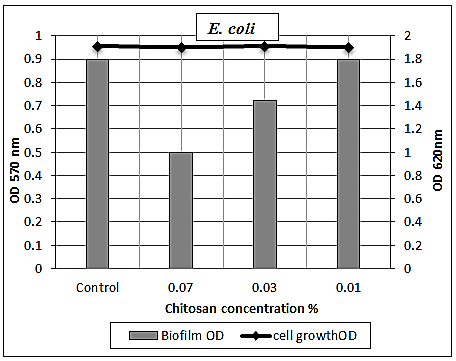 | Figure 10. Sub-MICs of chitosan against E. coli biofilm. Bacterial growth is linear chart, and the biofilm formation is column charts. The control is 0% chitosan concentraion |
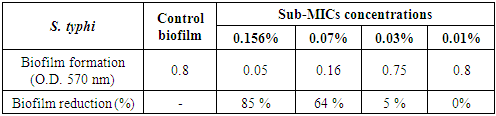
3.5.1.4. Salmonella typhi Biofilm
- The results presented in table (8) and illustrated in Figs. (7 & 11) showed the potential activity of chitosan against S. typhi biofilm. It was effective in breaking down the biofilm formed by this severe pathogenic microorganism especially at [1/2 MIC=0.156% and 1/4 MIC=0.07%] with biofilm reduction percentages were 85% and 64%, respectively. While, at concentration 0.03% of chitosan, the biofilm reduction percentage was 5% only.
|
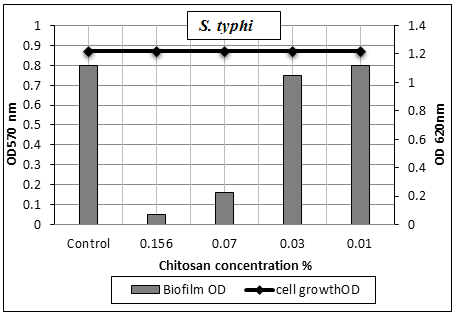 | Figure 11. Sub-MICs of chitosan against S. typhi biofilm. Bacterial growth is linear chart, and the biofilm formation is column charts. The control is 0% chitosan concentraion |
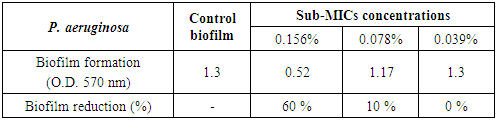
3.5.1.5. Pseudomonas aeruginosa Biofilm
- Chitosan had a mild effect on smashing up P. aeruginosa biofilm at sub-MICs. Since at concentrations 0.156% and 0.078%, the biofilms were 0.52 (O.D.) and 1.17 (O.D.), respectively, compared to the optical density of chitosan free culture, which was 1.3 (O.D.) with biofilm reduction percentages 60% and 10%, respectively (Table 9 and Figs. 7 & 12).
|
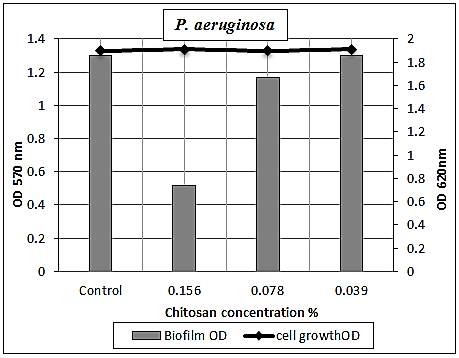 | Figure 12. Sub-MICs of chitosan against P. aeruginosa biofilm. Bacterial growth is linear chart, and the biofilm formation is column charts. The control is 0% chitosan concentraion |
3.6. Time Kill Assay
- The results illustrated in Fig. (13) exhibited the effectiveness of the bactericidal activity of chitosan at 1.25% for S. aureus and 0.312% for P. aeruginosa depending upon the Minimum Bactericidal Concentration (MBCs) for tested bacteria at interval periods of time. Since a decrease in O.D. values through time was found in the mixtures of chitosan-treated culture, and the bactericidal effect was observed during 3-6 hrs for S. aureus, while at 0-3 hrs for P. aeruginosa compared by the O.D. of untreated cultures. According to these data, the exposure time required by chitosan as a biocide is a short time, and this may be due to the greater cidal effect of chitosan.
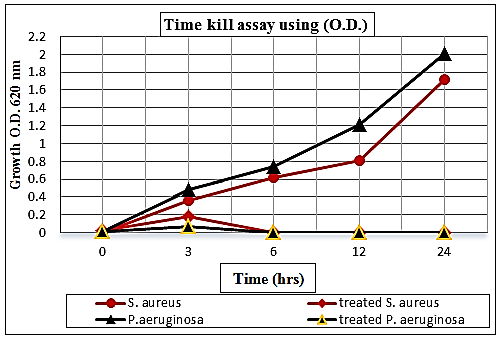 | Figure 13. Curve of time kill assay of chitosan against S. aureus and P. aeruginosa held at different time intervals (0, 3, 6, 12 and 24 hrs) compared to control growth |
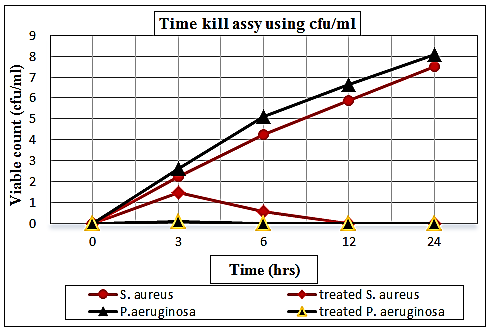 | Figure 14. Curve of viable count (log no. cfu/ml) of S. aureus and P. aeruginosa held at different time intervals (0, 3, 6, 12 and 24 hrs) supplemented with chitosan comparable to controls |
3.7. Morphological Changes of Chitosan-Treated and Non-Treated Bacteria via SEM
- By exposure to chitosan at MIC levels underwent considerable morphological alterations in comparison to the control were observed by a scanning electron microscope. S. aureus and P. aeruginosa showed similar patterns of altered morphology. The results indicated that important variables, including the elongation and an increase in cell wall roughness and a cluster of particles distributed along cell wall periphery surrounding the walls of treated cells, were observed. Moreover, both types of the cells were destroyed and ruptured (Fig. 15 A & B). Also, a clear alteration in the morphology of the cell wall with an increase in roughness and amorphous mass was observed (Fig. 15 A & B). The cell wall of both tested bacteria showed complete disruption, anomalous shape, so the cells appeared to be shrunk and even some were empty, and the remains were flaccid (Fig. 15 A). Furthermore, most of them appeared to be stuck together and melted. As well as, the elongation of cells was observed and associated with the disaggregation of grape like cluster and the cells showed deformation on their surface (Fig. 15 A). On the other hand, the untreated Gram-positive and Gram-negative cells (controls) appeared to be intact, separated from each other, turgid, and complete with smooth surface, regular and smooth thick cell walls suggesting that the cells are in a normal condition (Fig. 15 C1 & C2).
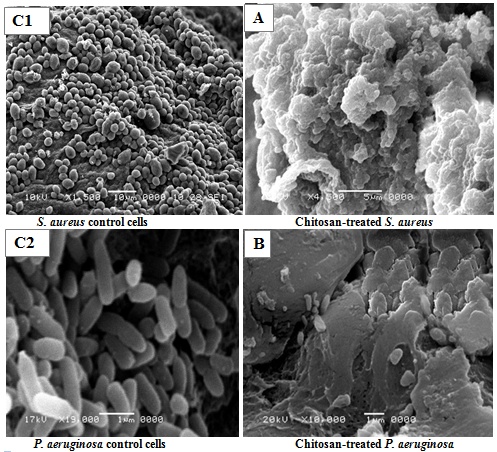 | Figure 15. Scanning electron micrographs (SEM) of untreated (controls; C1 & C2) and chitosan-treated S. aureus (A) and P. aeruginosa (B) |
4. Discussion
- Food is an essential commodity for nutrition and growth of the human being and also an excellent medium for the growth of various microorganisms. WHO has called for minimal usage of traditional preservatives in foods. In recent years, consumers are more particular about health and are concerned regarding the detrimental effects of food additives, and are also increasingly attractive towards foods which are more natural with no added chemical preservatives [15]. Thus, changes in consumer's preference and government policies challenged the food scientists to search for alternatives to chemical preservatives to improve the quality and extend the shelf-life of foodstuffs. Consequently, antimicrobials compounds derived from animal sources drawn attention as alternatives for chemical food preservatives. This study gives an overview of such antimicrobial along with its potential for elimination or control of undesirable microorganisms in the food to ensure its safety and long lastingness still an issue of concern. In that regards, much attention has been focused on the safety and efficiency of chitosan from the animal origin as the natural antimicrobials. The antimicrobial activity of chitosan has been observed in a wide variety of microorganisms, including bacteria, viruses, yeast, and fungi (Rabea et al., 2003) [5].In this study, chitosan showed antibacterial activities against Gram-positive, and Gram-negative tested foodborne bacterial isolates including B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa. However, chitosan had broad spectrum antibacterial activities, but the Gram-negative wall type was more sensitive.Regarding Gram-positive bacteria, chitosan markedly inhibited the growth of bacteria tested with the respective diameters zone of inhibition were narrower than the hallos obtained against Gram-negative bacteria. This trend of results is in agreement with Dutta et al. [16] and Wang et al. [17], they investigated and confirmed the antibacterial properties of chitosan. Also, Ganan et al. [18] investigated the antimicrobial activities of chitosan, which have been demonstrated against foodborne Gram-positive and Gram-negative bacteria. Also, these results are in accordance with findings of Wang et al. [17], they found that chitosan has been shown to inhibit both Gram-positive and Gram-negative bacteria, including Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Escherichia coli, Pseudomonas aeruginosa, Shigella dysenteriae, Vibrio spp. and Salmonella typhi. Also, these results are in harmony with the results obtained by No et al. [19], who found that chitosan between 28-1671 kDa inhibited the growth of both Gram-negative (E. coli, Pseudomonas fluorescens, Salmonella typhi, and Vibrio parahaemolyticus) and Gram-positive (Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, Lactobacillus plantarum, Lactobacillus brevis and Lactobacillus bulgaricus) bacteria.Our findings are consistent with the findings by Devlieghere et al. [20], their study demonstrated that Gram-negative bacteria were very susceptible to the effects of 43 kDa chitosan and the Gram-positive bacteria varied greatly in response to the chitosan with some being inhibited.Antibacterial properties of chitosan have been widely investigated and confirmed [16]. However, there are several mechanisms that have been proposed to explain the antimicrobial mechanisms of chitosan. The first proposed antibacterial mechanism of chitosan occurs due to electrostatic interactions between the chitosan amine group and the negatively charged components (lipopolysaccharides and proteins) of the outer cell distorting the cellular membrane and leakage of intracellular material [21]. This interpretation is in accordance with findings of Liu et al. [22], they treated S. aureus and E. coli with 0.5, and 0.25% chitosan (78 kDa) and the results showed that chitosan causing a reduction after 5 min, complete inactivation of E. coli after 120 min. On the other hand, S. aureus showed leakage of intracellular material and new cells were found to form without membranes or cell walls on the outside when examined by transmission electron microscope. The second suggestion attributed to chitosan may interrupt the physiological activities of the cell but affects Gram-positive and Gram-negative cells differently. They proposed that higher molecular weight chitosan form a polymer membrane to prevent nutrients from leaving and entering the cell on Gram-positive organisms, while lower molecular weight chitosan enters the Gram-negative cell binding to electronegative substances, which causes flocculation in the cytoplasm and disruption of physiological processes [23]. The third proposed mechanism of action is the inhibition of mRNA and protein synthesis by penetrating the cell of the microorganism and binding with DNA [16]. Another proposed antibacterial mechanism of chitosan is the chelation of essential nutrients needed for growth [16]. This proposed is in harmony with Kong et al. [24], they found that chitosan microspheres of 1456 kDa were chelating Mg2+ of the E. coli outer membrane, causing destabilization of the cell. The polycationic nature of chitosan (pKa = 6.3) is a prerequisite for antimicrobial activity. As pH is below the pKa of chitosan, electrostatic interaction between the polycationic structure (NH3+ groups of glucosamine) and the predominantly anionic components of the microorganisms surface (such as Gram-negative lipopolysaccharides and cell surface proteins) plays a significant role in the antimicrobial activity of chitosan. Eventually, the interaction between the positively charged NH3+ groups and the negatively charged microbial cell surface contribute to the leakage of protein and other intracellular components of the microbial cells, ultimately resulting in the impairment of vital bacteria activities [24, 25]. The number of amino groups linking to C-2 on chitosan backbones is important in electrostatic interaction, which indicates that a large amount of amino groups are capable of enhancing the antimicrobial activity. Therefore, chitosan with higher DD shows a stronger inhibitory effect than that of a lower DD chitosan [24]. Generally, the control of such pathogens like, B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa, which can cause life-threatening infections in humans causing outbreaks, and foodstuff spoilage using chitosan, thus considered as a promising natural antimicrobial agent for food preservation instead of hazardous of chemical ones.Chitosan has shown promising antifungal activity in vitro and in vivo, where they have been extensively studied against food associated fungi and yeasts. The obtained results showed the antifungal potential of chitosan against a range of tested fungi and yeast. From our findings, chitosan was demonstrated higher antifungal activity against A. niger, A. flavus, P. italicum, Rh. Stolonifer and C. albicans. Interestingly, chitosan exhibited strong anticandidal activity with complete growth inhibition. This inhibition activity was the strongest compared to amphotericin B and more recently its lipid formulations comprise the first line treatment of most fungi plus there are various side effects associated with the use of this antifungal agent. These results were in harmony with Mayachiew et al. [26], they reported the antifungal activity of chitosan. Furthermore, our results are in accordance with the findings of Badawy and Rabea [27], they used chitosan and chitosan derivatives at 1% concentrations to find that Botrytis cinerea and other fungal growth was reduced most likely due to chitosan causing a permeability change in the plasma membrane.Mechanisms of antifungal properties of chitosan were explained by Seyfarth et al. [28], they found that 120 kDa chitosan hydrochloride against Candida albicans, C. krusei, and C. glabrata disrupted the plasma membrane leading to permeable cells. A second proposed mechanism involves the accumulation of chitosan in the cell wall to inhibit growth [29]. El Ghaouth et al. [29] demonstrated that chitosan caused leakage of amino acids as well as morphological changes due to the accumulation of chitosan in the cell wall of Rhizopus stolinfer and B. cinerea. The third proposed mechanism involves the chelation of Ca2+, Zn2+, and other essential minerals needed for growth [30]. Also, Roller et al. [30] showed that chitosan reduced the growth rates of Mucor racemosus by making Ca2+ and other essential minerals unavailable. The last proposed mechanism involves chitosan interfering with conformation or physical properties of DNA in Fusarium solani [31]. Also, Hadwiger et al. [31] found that 0.0002% of chemically cleaved chitosan applied to pea plants 24 hr in advance was able to protect against F. solani.The MIC is defined as the lowest concentration of a drug/compound that will inhibit the visible growth of an organism in vitro after overnight incubation [32]. Our results demonstrated that chitosan had been shown to inhibit both Gram-positive and Gram-negative bacteria, including B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa and the Gram-positive bacteria seems to be least sensitive, while the Gram-negative bacteria found to be the most sensitive in the current study. The MICs values were a concentration dependant against the previously tested bacteria. These lines of results are very matched with No et al. [6], which they observed that MICs of chitosan vary widely with bacteria, ranging from 0.005% to 1.5% (w/v) and has been shown to inhibit both Gram-positive and Gram-negative bacteria. Alo, our results are in accordance with Sanchez-Gonzalez et al. [36], they reported that chitosan with a concentration of 0.1% was required to inhibit E. coli growth in meat preservation. However, another showed that the lower concentration (0.0075%) of chitosan was enough to inhibit the E. coli growth. The agreement with our results obtained in those studies was observed by No et al. [6], which they reported that chitosan (0.1%) was more effective in inhibiting Gram-positive than Gram-negative bacteria. Also, similar results have been reported by Kumar et al. [34], however, achieved complete growth inhibition of B. cereus with 0.01% of low MW chitosan within one hour, needing up to five hours to reach a comparable effect on E. coli. A similar trend was obtained for S. aureus and E. coli by the findings of Liu et al. [35], where their study demonstrated that chitosan of 78 kDa at 0.25 and 0.5% found that 0.5% chitosan showed more membrane damage of S. aureus and E. coli than 0.25%. Another study demonstrated that chitosan had been demonstrated to disrupt outer membranes of S. typhi at 0.01- 0.025% [21]. However, discrepancies in the results obtained by another study, chitosan had stronger antimicrobial activity against S. aureus than S. enterica and V. vulnificus, suggesting that chitosan is more efficient at inhibiting Gram-positive than Gram-negative bacteria [36].According to the results obtained by the current study, chitosan showed strong antifungal activity with low MICs values. These results are in a positive relationship with the other findings by Park et al. [37], they reported that low molecular weight (LMW)-chitosan showed strong antifungal activity against various pathogenic yeasts and hyphae-forming fungi at deficient concentrations. Further, our results are in harmony with the results obtained by Mohamed and Fahmy [38], they studied the antifungal action of a chitosan hydrogel on A. fumigatus and A. niger. They obtained a MIC value of 250 μg/ml for A. fumigatus and a MIC value of 125 μg/ml for A. niger. Also, other studies were in accordance with our findings in regards to antifungal activity of chitosan and its derivatives, where most of the clinical strains were inhibited within the range of 4.8 and 2,500 mg/l LMWC [37, 39]. Also, similar results have been reported by Tayel et al. [40], they reported a MIC of 1.25 mg/ml of chitosan against C. albicans. Also, another study in accordance with the findings by Seyfarth et al. [28], where their study demonstrated that chitosan concentration from 0 to 1.0% had been shown to increase the antifungal activity of chitosan against Botrytis cinerea, Candida albicans, C. krusei, C. glabrata, and Penicillium expansium. Some reports have suggested that, the polycationic character of chitosan is the most important mechanism of antifungal activity, as the cationic groups would interact with anionic components (i.e., proteins, phospholipids), of the cell wall of the fungus, and destabilize the membrane and interfering with the normal growth and metabolism of the fungal cells [37].The obtained results showed that all tested bacterial isolates exhibit strong potential for biofilm formation. P. aeruginosa which called standard Gram-negative biofilm producing bacteria was showed the strongest biofilm formation. These results are in accordance with Donlan [41], who assess the ability of the microorganisms attaches to surfaces in food processing and develop biofilms. Faille et al. [42] concluded that Bacillus strains were often isolated from biofilms in the food industries. Attached microorganisms are more resistant to sanitation chemicals than are their detached counterparts. This resistance is due to protection from organic materials and the extracellular polymeric substance (EPS) layer, which prevents chemicals from entering the biofilm or causes inactivation of the sanitizer [41, 43].Interestingly, reduced levels of biofilm formation in the presence of sub-inhibitory concentrations of chitosan were observed in the current study. Doses of sub-MICs showed a weak to moderate and strong influence against B. cereus, S. aureus, E. coli, S. typhi and P. aeruginosa biofilms. Chitosan has a track record for its inherent antimicrobial properties against a broad spectrum of organisms [44, 45]. Also, chitosan exhibits anti-biofilm activities, and the ability of chitosan to damage biofilms formed by microbes has been documented [46, 47]. Our results agree with most researchers’ findings these achieved in those studies were observed by Carlson et al. [48], they achieved a 5.5 log reduction in the attached biofilm of S. epidermidis using chitosan. Further, our results match with Orgaz et al. [47], which they studied the effectiveness of both medium molecular weight (MMW) chitosan and its enzymatically hydrolyzed against mature biofilms of four pathogenic strains, L. monocytogenes, B. cereus, S. aureus and S. enterica, and a food spoilage species, P. fluorescens.Chitosan exhibits anti-biofilm activities by several mechanisms involved chitosan has been shown to penetrate biofilms due to the ability of cationic chitosan to disrupt negatively charged cell membranes as microbes settle on the surface [48, 49]. Information on the physical and physicochemical properties of the biofilm matrices of different organisms, such as mess size and elasticity, amount and distribution of charges or polymeric junctions, or other properties affecting diffusion rates and retention abilities, is unfortunately very scarce [50]. Some exopolysaccharides such as those of Pseudomonas are known to be polianionic [51, 52], whereas others such as the adhesins of Staphylococcus are policationic [50]; this could be related to the response of their respective biofilms to chitosan exposure.Kill-time studies were performed to gain a better insight on bacteriostatic or bactericidal effects of the chitosan. The chitosan used in the present study appeared to be particularly effective, as bactericidal effects depending upon the MBCs for tested bacteria (S. aureus and P. aeruginosa). Based on our results the effectiveness of the bactericidal activity of chitosan at 1.25% and 0.312% for S. aureus and P. aeruginosa, respectively, were observed during 3-6 hrs for S. aureus, while at 0-3 hrs for P. aeruginosa compared by the O.D. of untreated solution. As well as, in chitosan-treated plates the counts of the two pathogens not detected by exposing to MBCs after 6 hr for S. aureus and between 0-3 hrs for P. aeruginosa at 37°C. In accordance with our results, Liu et al. [22] observed that, treated S. aureus and E. coli with 0.5 and 0.25% 78 kDa chitosan causing a 1 log reduction after 5 min, complete inactivation of E. coli after 120 min, and no change in S. aureus after 120 min. Furthermore, a similar level with our results have been reported by Kumar et al. [34], their study achieved complete growth inhibition of B. cereus with 0.01% of low MW chitosan within 1 hr, needing up to 5 hr to reach a comparable effect on E. coli.Scanning Electron Microscopy (SEM) was conducted to get the images of morphological damages in selected tested bacteria to confirm the antimicrobial activity of chitosan against foodborne related bacteria, including both Gram-positive (S. aureus) and Gram-negative (P. aeruginosa). These images directly illustrate the destructive effects of the chitosan on the tested bacteria. There are many possible explanations for the observations in harmony with our current study [15, 53]. The literature suggests that the active components might bind to the cell surface and then penetrate to the target sites, possibly the phospholipid bilayer of the cytoplasmic membrane and membrane-bound enzymes [15, 53, 54]. The SEM images show that some cells present damage as pores or deformity in the cell walls. Also, some authors have suggested that the damage to the cell wall and the cytoplasmic membrane was the loss of structural integrity and the ability of the membrane to act as a permeability barrier [55, 56].The findings of the present study thus, suggest that chitosan could be utilized effectively as an antimicrobial agent. This study will be the source for the possible discovery of new natural food preservatives for the treatment of foodborne pathogens.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML