-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(2): 39-44
doi:10.5923/j.microbiology.20170702.03

Evaluation of Antimicrobial Activity and Phytochemical Analysis of Thaiti Lemon Peels (Citrus latifolia Tanaka)
Racowski I., Piotto J., Procópio V., Freire V. T.
Laboratory of Microbiology, Faculdade de Tecnologia Termomecanica (FTT), São Bernardo do Campo, São Paulo, Brazil
Correspondence to: Racowski I., Laboratory of Microbiology, Faculdade de Tecnologia Termomecanica (FTT), São Bernardo do Campo, São Paulo, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The search for new antimicrobial compounds is ongoing. Its importance can be overemphasized in an era of emerging food borne diseases and resistant pathogenic organisms. The food industries show interest for the essential oils found in medicinal, aromatic and seasoning plants, as they contain antimicrobial activity against the decaying and/or pathogenic bacteria. The present study was carried out to find out the antimicrobial activity of ethanolic, cold and hot water extracts of Thaiti lemon peels (Citrus latifolia Tanaka) as well as commercial lemon essential oil. Antimicrobial analysis was done by using agar well diffusion method against bacterial pathogens and fungi. The results revealed the presence of tannins, flavonoids, and terpenoids in Thaiti lemon peels. Commercial essential oil of lemon peels exhibited the maximum zone of inhibition against Sacch. cerevisiae whereas alcoholic extract of lemon peels exhibited least zone of inhibition. Hot water extract exhibited maximum inhibition zones against E. coli but commercial essential oil gave least inhibition zone and to S. aureus all extracts (alcoholic and aqueous) and commercial essential oil exhibited the smallest zones of inhibition.
Keywords: Thaiti Lemon peel, Alcoholic extract, Aqueous extracts, Antimicrobial analysis, Phytochemical analysis, Inhibition zone
Cite this paper: Racowski I., Piotto J., Procópio V., Freire V. T., Evaluation of Antimicrobial Activity and Phytochemical Analysis of Thaiti Lemon Peels (Citrus latifolia Tanaka), Journal of Microbiology Research, Vol. 7 No. 2, 2017, pp. 39-44. doi: 10.5923/j.microbiology.20170702.03.
Article Outline
1. Introduction
- The concern with the consequences of the use of chemical preservatives in foods, either due to their uncontrollable use or because they might be considered responsible for carcinogenic and teratogenic attributes, is one of the factors that is being increasingly discussed among consumers [30]. Citrus latifolia belongs to Rutaceae family, common name is Tahiti lemon and this originated from South East Asia, probably in India or Southern China. Lemon is green, elliptically shaped berry fruit. Citrus fruit in general contain sugar, poly-saccharide, organic-acid, lipids, carotenoids, vitamins, minerals, flavonoids, bitter lemonoids and volatile compounds [34].Lemon is a product easy to be found and produced throughout the year, and Brazil is one of the world’s largest producers [23]. Thaiti lemon variety (Citrus latifolia Tanaka), also known as Green Lemon is a fruit of tropical origin, and has great economic importance for Brazil which produced 1.169.370 tons of lemon in 2013 [18].This lemon, which is actually an acidic lime, has smooth and thin peel that is easily separable from the pulp [7, 37]. Tahiti acid lime pulp has around 10 segments with axis, which are vesicles that contain juice, also known as "bagasse". This structure has hemicellulose, pectic substances, cellulose, sugars, flavonoids, minerals and vitamins. The juice contained in these vesicles represents about 50% of the Tahiti lemon weight, and has ascorbic acid content between 20 and 40 mg / 100 ml [7, 8]. This fruit is widely used in Brazil, whether fresh in popular cookery or in the production of industrialized juice. In this case, only 50% of the fruit is used, generating a large amount of industrial residue [38].These residues represent a problem for management, pollution, and environmental issues, due to microbial spoilage [22, 29].Fruit peel waste are highly perishable and seasonal, is a problem to the processing industries and pollution monitoring agencies [2]. The fruit peels are rich in nutrients and contain many phytochemicals; they can be efficiently used as drugs or as food supplements too [6]. Citrus peels are employed for a variety of uses, as fodder at fisheries, activated carbon, raw materials for traditional paper [19]. The essential oil is also obtained from the peel, which is highly valued, with wide use in the pharmaceutical industry and cleaning products [27]. Citrus fruit products are known to potent antimicrobial agents like, bacteria, fungi [28]. The antimicrobial activity of plants had been received attention many years ago as one of the most effective mechanism for the control of microorganisms [21]. Among these microorganisms may be highlighted: Staphylococcus aureus (is one of the most common bacteria implicated in food poisoning [25]), Escherichia coli (is a naturally occurring bacteria in the intestinal tract of man. The acquisition of invasion factors increases their ability to adapt to new niches and their disease-causing abilities [1]) and Saccharomyces cerevisiae (yeast that performs fermentative processes in the food industry. Usually be retarded by weak organic acid preservatives, the inhibition often requires levels of preservative that are near or greater than the legal limits [16]).The aim of this study was to verify the antimicrobial properties of Tahiti lemon peel extracts (alcoholic and aqueous) against bacterial (Escherichia coli and Staphylococcus aureus), and yeast (Saccharomyces cerevisiae), and their phytochemical analysis which are responsible for antimicrobial activity.
2. Method and Materials
2.1. Sample Collection
- Thaiti lemons (Citrus latifolia Tanaka) were purchased from retail chains in the city of São Bernardo do Campo, SP, Brazil. The maturation stage was visually verified, and mature lemons with intact peels were selected. Commercial essential Thaiti lemon oil (Citroflavor©) was purchased from company (Citroflavor©, Brazil), the methodology used for the oil extraction, according to the company's website, is the cold pressing of peels.
2.2. Test Microorganisms
- E. coli and S. aureus were acquired isolated and selected in a test tube containing nutrient agar (Merck®, Germany), while Sacch. cerevisiae used was freeze-dried (Fleischmann®, United States), rehydrated and maintained on a Petri dish with Sabouraud Dextrose Agar Medium (Merck®, Germany) until use. These microorganisms were stored at 10°C at the Laboratory of Microbiology of the Faculty of Tecnologia Termomecanica (FTT) after growth. To assure the purity and viability of the young culture, the bacteria studied were cultured in Nutrient Agar in duplicate during the week of use for 24 and 48 h at 37°C and the yeast was grown in Sabouraud Dextrose Agar, also in the same week of use in duplicate and in the same period of time, but at 28°C. To prepare the inoculum used in antimicrobial plaques, the methodology used was described by Piotto and Procópio [36], where five colonies of each microorganism were selected, each with approximately 1 mm in diameter, which were suspended in 5 mL of sterile 0.85% NaCl and homogenized in a tube shaker for 15 s. The inoculum density was adjusted with 0.85% sterile saline solution and determined by spectrophotometry at 520 nm to obtain 95% transmittance, obtaining a final concentration between 1×106 and 5×106 cells / ml [13, 31].
2.3. Preparation of Thaiti Lemon Peel Extracts
- After selection, lemons were washed in running water and peeled. Extracts were obtained according to methodology described by Hussain et al. [17] with some modifications, in which lemon peels were oven dried for 2 days at 30°C. After this period, peels were ground in a mortar previously sterilized with 70% alcohol until a powder was obtained. The powder obtained was placed in contact with two different solvents: 96% alcohol and distilled water (an extraction carried out at room temperature and another at water boiling temperature) at proportion of 10% (w/w), using 26 g of lemon peel powder for 260 g of solvent, which was kept immersed in the solvent for 24 h and this solution was packed in a clear glass vial wrapped with aluminum foil. After this time, extracts were filtered on sterilized Whatman No.1 filter paper and the resulting filtrates were concentrated in a vacuum rotary evaporator at 40°C for the alcoholic and the aqueous extracts were concentrated in lyophilizer (L101, Liobras, Brazil) until all water was removed to form a powder again. The lyophilized products were used to make a solution by the addition of 20 ml of distilled water and together with the oily solution resulting from the rotovaporator (RV 10 Digital, Ika, German) were used to check the antimicrobial effect. After obtaining extracts, the pH of all, as well as that of the essential oil, were measured with the aid of bench pH meter (UB-10, Denver Instrument, United States) calibrated with buffer solutions of pH 4.0 and 7.0 [3].
2.4. Antimicrobial Assay
- This was carried out using the protocol described by Olivares et al. [33]. Test organisms were subcultured onto fresh suitable broth medium. Broth cultures were then incubated at 37°C and 28°C, the methodology used was described by Piotto and Procópio [36]. Mueller–Hinton agar was used as bacterial medium and Sabouraud agar as fungal medium. Wells of 6 mm in diameter were punched in the culture media with sterile cork borer. The extracts were there after used to fill the boreholes. Each plate was kept in the refrigerator at 4°C for 1 h before incubating at 37°C for 24 h (bacteria) and 72 h (fungi). Zones of inhibition around the wells, measured in millimeters, were used as positive bioactivity. From the different treatments, solutions at 5, 10, 15, 20 and 25% were prepared with sterile distilled water (w/w). The 0 and 100% controls are respectively pure sterile distilled water and extract obtained without any dilution (100%) and also samples of distilled water acidified by the addition of 0.01M citric acid so as to result in pH equal to that of solutions. This was done to verify if the inhibitory power would be related to the acidity of extracts or to substances present in them. Thus, after inoculation, plates were incubated in an oven according to the optimum temperature of each microorganism studied: bacteria at 37°C and yeast at 28°C, and observed in 24h for the measurement of zone of inhibition. The diameter of zone of inhibition around each well was measured with the aid of a digital caliper, each inhibition zone being measured in triplicate (in three different ways to reduce error due to irregular growth) and submitted to Kruskal Walis analysis of variance and compared by the Mann Whitney test, both with 5% significance using the Action Stat software (Version 3.1, Estatcamp, Brazil).
2.5. Phytochemical Analysis
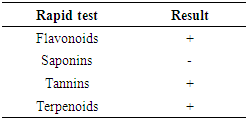
- Phytochemical are the main constituents of any plant sample, which are responsible for secondary metabolites also. Rapid tests were performed according to methodology of Pathak et al. [35]. Tannins: Take 5 ml of lemon peel filtrate and add 5 ml distilled water then heat at 80-100°C for 10 min in water bath, then filter it after that add 1% ferric chloride (5-6 drops). Dark green color indicates the presence of tannins. Saponins: Take 2 g of powdered lemon peel sample were boiled in 20 ml of distilled water and filtered on filter paper No. 102. About 10 mL were collected and mixed with 5 mL of distilled water in a test tube that was vigorously shaken until persistent and stable foam was obtained. The foam formed was mixed with 3 drops of olive oil. The formation of an emulsion would indicate the presence of saponins. Flavonoids: A few drops of 1% NH3 solution were added to lemon peel extract in a test tube. If there was a color change to yellow in the resulting solution, it would indicate the presence of flavonoids. Terpenoids: 5 mL of lemon peel extract was mixed with 2 mL CHCl3 in a test tube. These tubes were added of 3 mL H2SO4 and the formation of a reddish-brown staining interface was verified, indicating the presence of terpenoids.
3. Results and Discussion
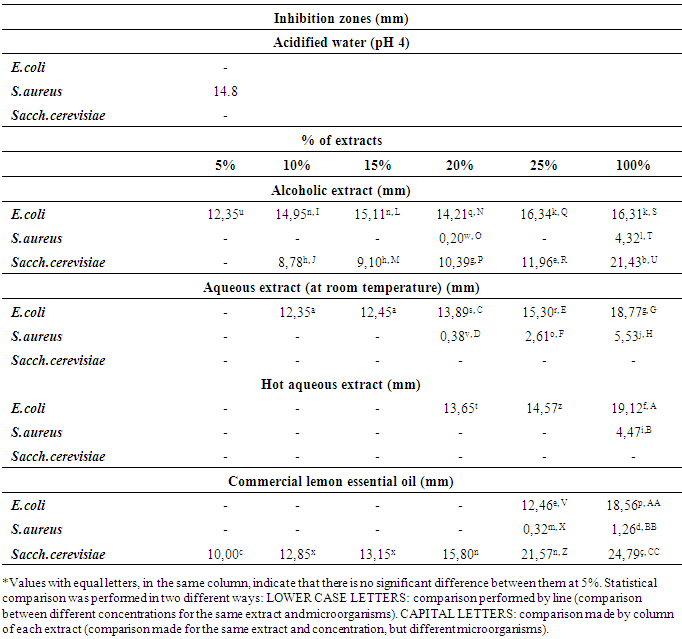
- The antimicrobial activity of Thaiti lemon peel extracts against both Gram-positive, Gram-negative bacteria and yeast (Table 1) was investigated. The inhibition zones (mm) of the Thaiti lemon peel extracts on selected microorganisms are reported in Table 1. Sterile distilled water at pH 4, used as negative control. The results of the inhibition zones for Sacch. cerevisae, E. coli and S. aureus are presented in Table 1.
|
|
4. Conclusions
- It was concluded that Thaiti lemon peel contains Bioactive substances that inhibit the growth of microorganisms, but the inhibition efficiency is related to the extraction process: E. coli and S. aureus were more inhibited when processed by alcoholic and aqueous route (both at room and hot water temperature extraction). For Sacch. cerevisiae, aqueous extracts were not efficient for inhibiting their growth, unlike when using alcoholic extract. The essential oil was efficient in inhibiting the three microorganisms under study. E. coli and Sacch. cerevisiae, the essential oil showed the best inhibition, followed by hot aqueous extract and aqueous extract obtained at room temperature and finally by alcoholic extract. In the case of Sacch. cerevisiae, the second best extract was the alcoholic extract, emphasizing that the aqueous extracts could not inhibit its growth. For S. aureus, the result was quite different, where statistically there would be no significant differences at 5% level, growth inhibition by alcoholic and aqueous extracts, and these showed better inhibition compared to commercial essential oil. It is noteworthy that, in general, yeast and Gram negative bacteria were more sensitive than Gram positive bacteria, and it could be assumed that this is related to the mechanism by which inhibition occurs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML