-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(2): 23-30
doi:10.5923/j.microbiology.20170702.01

Antimicrobial, Anti-plasmodial and Cytotoxicity Properties of Bioactive Compounds from Fusarium sp. USNPF102
Koochana Pranay Kumar1, Kalpana Javvaji1, Yedla Poornachandra1, Aparna Devi Allanki2, Sunil Misra1, 3
1Pharmacology and Toxicology Division, CSIR-Indian Institute of Chemical Technology, Hyderabad, India
2CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India
3Academy of Scientific and Innovative Research (AcSIR), India
Correspondence to: Sunil Misra, Pharmacology and Toxicology Division, CSIR-Indian Institute of Chemical Technology, Hyderabad, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study aims at isolation and identification of bioactive compounds from the soil borne Fusarium fungal species. Based on both morphological characteristics and internal transcribed spacer (ITS) regions of rDNA, this fungus was identified as Fusarium sp. USNPF102 with Accession No: HQ223338. From Ethyl acetate extract of this fungus, three compounds namely Javanicin (1), Fusarubin (2) and 3-O-methyl Fusarubin (3) were isolated and validated with the detailed chemically characterization using spectroscopic data (IR, LCMS, 1H & 13C NMR and DEPT). The bioefficacy investigations revealed that compound (2) showed a broad spectrum antimicrobial activity against S.aureus,S.epidermis,E.coli,P.aeroginosa,K.pneumoniae,C.albicansandC.rugosa. The compound (3) showed an excellent plasomodial activity against P.falciparum 3D7 strain with an IC50 value of 36.67µM with < 15% hemolytic activity, which is one of the main features of a compound as potent antimalarial agent. The invitro anticancer efficacy of these three compounds revealed that, compound 2and 3 inhibited the cell viability with an IC50 value ranging from 1-15µM against all the six cancer cell lines. Hence, present results could be led to further research towards the design of compound 2 and 3 as cancer chemotherapeutic agents and 3 as a potent antimalarial agent.
Keywords: Fusariumsp.USNPF102,Javanicin (1), Fusarubin (2) and 3-O-methyl Fusarubin (3), Antimicrobial, anticancer, Antimalarial activity
Cite this paper: Koochana Pranay Kumar, Kalpana Javvaji, Yedla Poornachandra, Aparna Devi Allanki, Sunil Misra, Antimicrobial, Anti-plasmodial and Cytotoxicity Properties of Bioactive Compounds from Fusarium sp. USNPF102, Journal of Microbiology Research, Vol. 7 No. 2, 2017, pp. 23-30. doi: 10.5923/j.microbiology.20170702.01.
Article Outline
1. Introduction
- Exploration for diverse chemical structure with pharmacological properties of secondary metabolites from fungal sources have been a promising area of research in recent decades [1, 2]. As a part of our recent initiative for isolation of newer bioactive molecules from soil fungi, Fusarium sp. USNPF102 was identified from the soil sample from Nallamala forest region, Talengana, India. This ascomycete Fusarium genus mostly known for their pathogenic to agricultural crops [3]. Apart from their pathogenic features, various Fusarium are also reported to produce some of the unique and beneficial metabolites such as Naphthoquinones and their derivatives, such as Javanicin, Fusarubin, Herbarin, Dihydro naphthalenone [4, 5] of pharmaceutical importance. These class of compounds have been attracted more and more interest due to their broad spectrum antibacterial [6], insecticidal [7], cytotoxicity [8-10], antimalarial [11], anti-HIV [12] properties. The Ethyl acetate (EtOAc) extract of this fungus led to isolation of three compounds viz., Javanicin (1), Fusarubin (2) and 3-O-methyl Fusarubin (3) which were later confirmed and chemically characterized. Although, these compounds were earlier isolated and reported from different species of Fusarium but for the first time all these three compounds (1-3) detailed here were obtained from the same Fusarium sp. USNPF102. Further, bioefficacy properties such as antimicrobial, antimalarial, and anticancer of these compounds were assessed in detail.
2. Materials and Methods
2.1. Soil sample and Fungal Identification
- The Nallamala forests form a series of parallel hill ranges oriented North-South towards the Eastern portion of the peninsular India in the state of Telangana, India. This forest is situated between 15°30’-16°30’ N latitudes and 78°30’-80°10’ E longitudes is renowned for being rich in biodiversity [13]. The fungus was identified based on the morphological features and molecular characteristics [14] and confirmed as Fusarium sp. USNPF102 (Supplementary data Fig. 1, 2 and 3).
2.2. Fermentation
- The fermentation was carried out in liquid Potato dextrose broth (Himedia, Mumbai) in 1L Erlenmeyer flasks (300 mL/flask). Two discs (5mm) from a week old culture were inoculated into the autoclaved media under aseptic conditions. The flasks were then incubated at 27ºC for 10 days under static conditions for the production of metabolites. The mycelium was separated from the liquid media using Buchner funnel and EtOAc was added to the fermented media and flasks were kept under shaking condition for 5 hr. The organic layer (EtOAc) was separated and concentrated under reduced vacuum to yield a dark reddish coloured extract.
2.3. Isolation of Metabolites
- The EtOAc fractions of culture filtrates of Fusarium sp. was loaded on a column of silica gel (100-120 mesh) and eluted successively with solvents of increasing polarity (n-hexane, Ethyl acetate, Methanol) in different percentages based on the standard TLC. Structure elucidation of the purified fractions was further characterized using spectroscopic techniques (1H NMR & 13C NMR), DEPT, IR and (LC-MS and HRMS).
2.4. Chemical Characterization
- (1) Javanicin (PF-2) [5,8-dihydroxy-2-methoxy-6-methyl-7-(2-oxopropyl)naphthalene-1,4-dione]: Orange solid, mp. 207-208oC. Molecular Formula: C15H14O6; Molecular weight: 290.272; MS (m/e): 290, 248, 230, 219, 205; IR (KBR, cm1): 3424 (OH), 1603, 1717 (C=O); 1H (NMR, CDCl3): 2.24 (3H, s, CH3), 2.30 (3H, s, CH3), 3.91 (2H, s, CH2), 3.93 (3H, s, CH3), 6.21 (1H, s, H-6), 12.86 (brs, 1H, O-H), 13.26 (brs, 1H, O-H). 13C NMR (CDCl3, 75MHz): δ 12.8 (Ar-CH3), 31.9 (carbonyl attached CH3), 41.2 (CH2), 56.7 (O-CH3), 108.3, 109.6 (Quinone attached Ar-CH), 134.1, 142.4 (Ar-CH), 160.3, 160.5 (OH-Ar-CH), 161.4 (Quinone C), 177.6, 184.3, 203.7 (3- C=O). (2) Fusarubin (PF-5) [3,5,10-trihydroxy-7-methoxy-3-methyl-3,4-dihydro-1H-benzo[g]isochromene-6,9-dione]: Brick red solid, mp 218 oC; Molecular Formula: C15H14O7; Molecular weight: 306.27; MS (m/e): 306, 288, 273, 259, 246; IR (KBR, cm1): 3425 (OH), 1620, 1739 (C=O); 1H (NMR, 500 MHz, CDCl3) : δ 1.66 (3H, s, CH3), 2.27 (d, 1H, j = 2 Hz, OH), 2.70 - 2.74 (1H, d, J = 18 Hz, J = 2, CH2), 3.02 - 3.06 (1H, d, J = 18 Hz, CH2), 3.94 (3H, s, CH3-O), 4.89 (2H, s, -CH2-O-), 6.18 (1H, s, Quinone ring H-6), 12.69 (s, O-H), 12.69 (s, OH); 13C NMR (CDCl3, 75MHz): δ 22.54 (CH3), 32.42 (CH2), 56.63 (O-CH2), 58.2 (Quinone O-CH3), 107.4, 109.5 (Quinone attached Ar, Quinone ring carbon), 133.0, 137.1 (pryan ring attached Ar C), 156.7, 160.3 (OH attached Ar C), 160.6 (Quinone ring carbon).(3) 3-O-methyl Fusarubin (PF-4) [5,10-dihydroxy-3,7-dimethoxy-3-methyl-3,4-dihydro-1H-benzo[g]isochromene-6,9-dione]: Reddish orange needles, mp 157-158°C; Molecular Formula: C16H16O7; Molecular weight: 320.298; IR (KBR, cm1): 3447 (OH), 1652, 1729 (C=O), 1H (NMR, 600 MHz, CDCl3): δ 1.55 (3H, s, CH3), 2.99 – 3.05 (3H, dd, J = 1.8 Hz, J = 2.07 Hz, pyran ring CH2), 3.31 (3H, s, O-CH3), 3.95 (3H, s, Quinone O-CH3), 4.52 – 4.60 (2H, td, J = 18.12 Hz, pyran ring CH2), 4.84 – 4.91 (2H, dd, J = 17.75 Hz, pyran ring CH2), 6.17 (1H, s, Quinone ring H), 12.66 (1H, brs, O-H), 12.94 (brs, OH); 13C NMR (CDCl3, 75MHz): δ 22.8 (CH3), 32.97(CH2), 48.9(-O-CH3), 56.70 (O-CH2), 58.65 (Quinone O-CH3), 107.5, 109.5 (Quinone attached Ar, Quinone ring carbon), 132.85, 137.16 (pyran ring attached Ar C), 156.26, 160.66 (OH attached Ar C), 160.77 (Quinone ring carbon), 178.14, 184.68 (C=O) The structures of these 3 compounds were determined and confirmed by comparing with spectroscopic data with those in the literature [6].
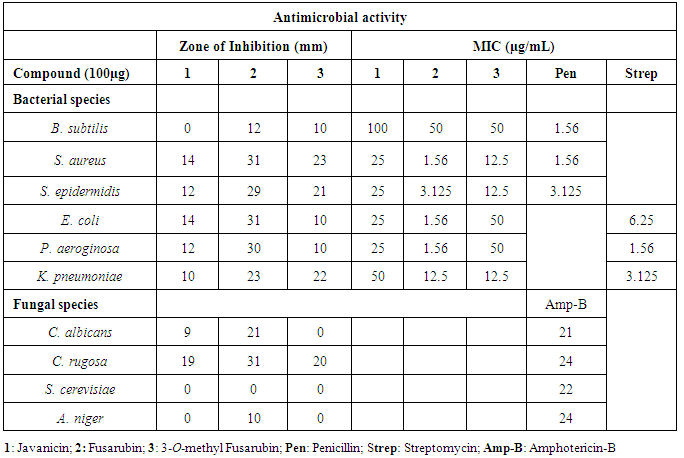
2.5. Bioactivity Studies (Antimicrobial Activity)
- In vitro antimicrobial activity of the compounds was studied against a panel of test organisms, Gram-positive viz., Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 96), Staphylococcus epidermidis and Gram-negative viz., Escherichia coli (MTCC 443), Pseudomonas aeruginosa (MTCC 741) and Klebsiella pneumoniae (MTCC 618); the fungal strains, Candida albicans (MTCC 227), Saccharomyces cerevisiae (MTCC 36), Aspergillus niger (MTCC 282) by Agar diffusion Method [15]. The suspension (400 µL) of the test bacteria and fungi which were spread on the nutrient agar and potato dextrose agar respectively. The disc (6 mm diameter) impregnated with 10µL of the isolated compounds (100µg) and DMSO (negative control) was placed on the inoculated agar, which was incubated for 24h at 37°C (bacteria) or 48 h at 25-27°C (fungi). Penicillin, Streptomycin sulfate (50µg/mL) and Amphotericin-B (50µg/mL) were used as the positive controls for bacteria and fungi respectively. Clear inhibition zones around the discs indicated the presence of antimicrobial activity. All the tests and analyses were carried out in triplicate.
2.6. Determination of Minimum Inhibitory Concentration (MIC)
- The minimum inhibitory concentrations of isolated compounds (1-3) were tested against a panel of bacteria strains by broth dilution method recommended by National Committee for Clinical Laboratory standards [16]. Penicillin and Streptomycin were used as standard agents for comparison. Plates were incubated at 37°C for 24h. Microbial growth was indicated by the presence of turbidity and a pellet at the bottom of the well and calculated the minimum inhibitory concentration (MIC).
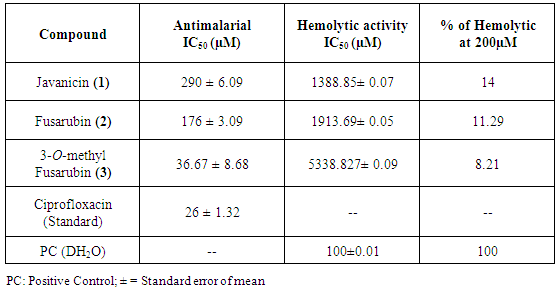
2.7. Antimalarial Activity
- The P. falciparum 3D7 strains were obtained from the Malaria Research and Reference Reagent Resource Centre (MR4). Ciprofloxacin from Sigma, cell culture reagents were procured from Lonza and Invitrogen, and SYBR Green 1 were purchased from Invitrogen.Parasite culture and growth inhibition assaysThe 3D7 P. falciparum strain were cultured in human red blood cells at 2% hematocrit in the presence of a gas mixture (5% CO2, 5% O2, and 90% N2) in RPMI 1640 medium containing 41.1mg/litre hypoxanthine, 300mg/litre glutamine, 2.5% human serum, and 0.5% Albumax II (4). Parasites were synchronized at the ring stage by treatment with 5% D-sorbitol [17]. 200 x stocks of compounds were made in DMSO or in 1N HCl (ciprofloxacin), and serially diluted 2 fold in 100 μl culture medium across rows of a 96 well tissue culture plate. Control wells contained DMSO (0.05%) or 500nM chloroquine. 100 μl of parasite suspension (1% ring-infected erythrocytes at 4% hematocrit) was added to each well, and plates were incubated in a modular incubator chamber (Billups-Rothenberg, Inc.) with the gas mixture at 37°C for 50 hours. 100μl culture medium was carefully removed from each well at the end of incubation, and the SYBR Green 1 dye in 100μl lysis buffer (20mM Tris-Cl, 5mM EDTA, 0.008% saponin, 0.08% Triton X-100, pH 7.5) was added to each well. The plate was incubated at 37°C for 30 min, and fluorescence was measured (Ex: 485 nm, Em: 530nm, gain setting: 50) using an Infinite M200 multimode microplate reader (TECAN) as described previously [18]. Fluorescence values of inhibitor-treated cultures were normalized as the percentage of DMSO-treated cultures, plotted against inhibitor concentrations, and analyzed using nonlinear regression analysis to determine IC50 concentrations for the inhibitors as described earlier [19].
2.8. In vitro Hemolytic Assay
- Hemolytic assay was carried out by adopting the method of Bulmus et al. (2003) [20]. Freshly collected Rat red blood cells were taken and washed three times with 150mM NaCl (2500 rpm for 10 minutes). The serum was removed and the cells were suspended in 100mM sodium phosphate buffer. The compounds are dissolved in DMSO and four different concentrations (10μM, 50μM, 100μM, and 200μM) of each compound were mixed with 200mL of RBC solutions and the final reaction mixture volume was made up to 1 ml by adding sodium phosphate buffer. The reaction mixture was then placed in water bath for 1 h at 37°C. After the incubation time the reaction mixture was centrifuged again at 2500 rpm for 15 minutes. The supernatant was collected and the optical density was measured at 541nm keeping sodium phosphate buffer as blank. Deionized water was used as a positive control. The experiment was done in triplicate and percentage of hemolysis were calculated.
2.9. Ethical Clearance
- Swiss albino mice of 3-4 week (approximately 20g) old were procured from National Institute of Nutrition (NIN), Hyderabad, India were acclimatized in our animal house at 23 ± 2°C for one week prior to experimentation. All animal studies were performed based on the approval by Institutional Animal Ethics Committee (IAEC Approval No. IICT / BIO / TOX / SM / 20/12/2013 / 09), CSIR-IICT, India.
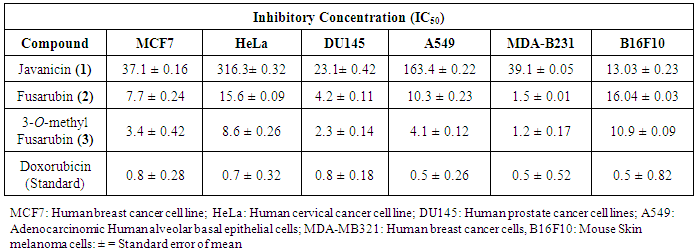
2.10. In vitro Cytotoxic Assay
- Cytotoxicity of the purified compounds at various concentrations was determined on the basis of measurement of in vitro growth inhibition of tumor cell lines in 96 well plates by cell-mediated reduction of tetrazolium salt to water insoluble formazan crystals and doxorubicin was used as standard anticancer agent for comparison. The cytotoxicity was determined by a panel of six tumor cell lines: MDA-MB-231 (Human breast adenocarcinoma cells, ATCC No. HTB-26), DU145 (Human prostate cancer cells, ATCC No. HTB-81), MCF7 (Human breast adenocarcinoma cells, ATCC No. HTB-22), HeLa (Human cervical cancer cells, ATCC No. CCL-2), B16-F10 (Mouse melanoma cells, ATCC No. CRL-6475), A549 (Human alveolar adenocarcinoma epithelial cells, ATCC No. CCL-185) and HEK 293a (Mouse Skin melanoma cells) using MTT assay [21]. The test compounds were solubilized in DMSO and the effect of test compounds on cell lines were measured at the wavelength of 540 nm using a multimode reader (Infinite® M200, Tecan, Switzerland). The IC50 values (50% inhibitory concentration) were calculated from the plotted absorbance data for the dose-response curves. IC50 values (μM) are indicated in the table as means ± SD of three independent experiments.
2.11. Anti-inflammatory Activity
- THP1 cell culture and Measurement of cell viabilityTHP1 monocytes were obtained from ATCC and grown in RPMI 1640 (Sigma) containing 10% FBS (Lonza), non-essential amino acids, penicillin (100U/mL), and streptomycin (100μg/mL) in 75-cm2 filter vent flasks (Costar), incubated at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The effect of isolated compounds on cell viability was determined by Trypan blue dye exclusion assay. THP1 cells were seeded at a density of 2 × 105 cells/mL in 24-well plates in triplicates and were treated with test compounds (10μM) for 48 h. The cells were harvested and re-suspended in 0.4% Trypan blue and cell viability were calculated.
2.12. Enzyme-linked Immunosorbent Assay for IL-1β and MCP-1 Inhibition
- To check the inhibitory effects of isolated metabolites on PMA induced inhibition of inflammation, THP1 monocytes were seeded at a density of 2 × 105 cells/mL in 24 well plates. The cells were pre-treated with 5, 10 and 20μM concentrations of compounds for 1h before they were stimulated with 10ng/mL of PMA. After 48 h of PMA stimulation supernatants were harvested and assayed for IL-1β, MCP-1 and TNF-α using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer's instructions (eBiosciences, San Diego, CA, USA). The absorbance in each well was measured with a microplate reader at 450 nm and corrected at 570 nm. Concentrations of cytokines released were obtained and the percentage of inhibition of cytokines production was calculated as compared to control conditions. IC50 was also obtained as a mean of anti-inflammatory potency, expressing the predicted concentration of the correspondent compound able to reach 50% inhibition. The standard curves were calculated by plotting the pg/mL concentrations versus absorbance values from the standards and were used to quantify the amount of cytokines released by the cells.
3. Results and Discussion
3.1. Isolation and Chemical Characterization of Metabolites
- The ethyl acetate extract of Fusarium sp. was subjected to silica gel column chromatography, eluted successively with different solvents in increasing polarity (Hexane to Ethyl acetate and Methanol) which resulted in the isolation of 3 fractions (Fractions 1–3). These fractions were later identified as Javanicin (1), Fusarubin (2) and 3-O-methyl Fusarubin (3) based on their detailed chemical characterization (Fig. 1).
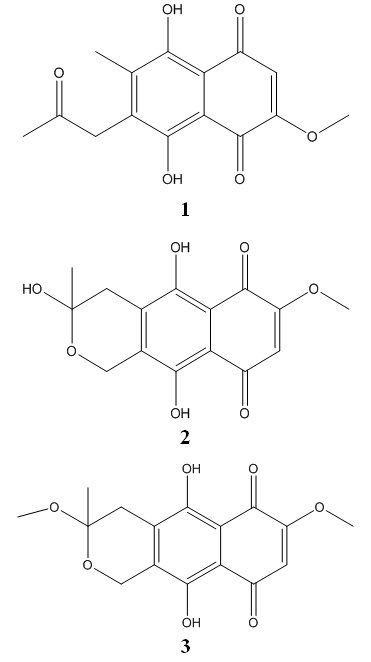 | Figure 1. Javanicin (1), Fusarubin (2), 3-O-Methyl Fusarubin (3) |
3.2. Antimicrobial Activity
- Many natural naphthoquinones and their derivatives were reported to show antimicrobial properties [22, 23]. Hence, in this study three naphthoquinone were obtained from Fusarium sp. USNPF102 were used to assess the antibacterial and antifungal properties. The zone of inhibition and MIC values of the compounds are represented in the Table 1. The compound 2 showed a broad spectrum antimicrobial activity against S. aureus, S. epidermidis, E. coli, P. aeroginosa, K. pneumoniae, C. albicans and C. rugosa with zone of inhibition 31, 29, 31, 30, 23, 21 and 31mm (at 100µg concentration) and their MIC values ranging from 12.5 - 1.56 µg/mL. The compound 2 showed moderate activity against B. subtilis with MIC values of 50µg/mL but inactive against S. cerevisiae and A. niger. The compound 3 displayed good activity against S. aureus, S. epidermidis, K. pneumoniae and C. rugosa with inhibition zone of 23, 21 22 and 20 mm with MIC value of 12.5µg/mL. The compound 1 showed a moderate activity against all the bacterial strain with MIC ranging from 25-50µg/mL concentration and good antifungal activity against C. rugosa. The antimicrobial properties of these three compounds (1-3) are also in agreement with the earlier reports on isolated naphthoquinones isolated from F. solani [6]. The antimicrobial activity of naphthoquinones may due to the presence of a substitution group at position 2 or 3, which is either an electron-releasing or withdrawing capability [24]. However, in this study, it is observed that, 7-methoxy, 3-methyl and 6,9-dione position of in compound 2 and 3 might be responsible for the antimicrobial activity.
|
3.3. In vitro Antimalarial and Hemolytic Activity
- All the isolated compounds (1-3) were tested for antimalarial activity against P. falcipuram (3D7) strain. IC50 values along with standard deviation of the compounds and standard Ciprofloxacin are represented in the Table 2. Among all the tested compounds, compound 3 showed significant activity with IC50 value of 36.67µM concentration in comparison with the standard (Ciprofloxacin) value of 26µM. Other two compounds (2 and 1) showed higher IC50 values of 176 µM and 290µM respectively. Previously, these naphthaquinone derivatives isolated from sea fan derived fungi Fusarium spp. PSU-F14 and PSU-F135 were also reported for antimalarial activity against P. falciparum K1 strain [11]. It is well established that naphthoquinones types of compounds found in the plants are being widely used in China and South America to control malignant as well as some parasitic diseases such as malaria parasite [24]. Although, the mechanism of action of naphthoquinones as an antiplasmodial activity has not been completely understood but these type of compounds mostly show some degree of antiplasmodial activity by interfering with the electron transport chain of mitochondria [25] which in turn inhibits the nucleic acid and ATP synthesis [26]. Naphthoquinone were also reported to show the antiparasitic effect by increasing the production of reactive oxygen species like superoxide anion and hydrogen peroxide in Trypanosoma cruzi [27]. Among these three compounds, the compound 3 (3-O-methyl Fusarubin) exhibited a good antiplasmodial activity against P. falciparum with least haemolytic activity. As per USFDA standards the compound that shows a good antiplasmodial activity and with low hemolytic activity can be considered as potential antimalarial drug [28, 29]. Percentage of hemolysis in presence of compound (1-3) are shown in Table 2. All the compounds at 200µM were found to be non-toxic to the RBC with < 15% of hemolysis. The compound 3 showed less toxic with 8.21% at 200µM which can be categorized as a low hemolytic activity. The compound 1 and 2 also displayed 14 and 11.29% of hemolysis respectively. In this study also, the compound 3, showed both potent antiplasmodial activity with a least hemolytic activity confirms as a good antimalarial agent.
|
3.4. Anti-inflammatory Activity
- The isolated compounds (1-3) were screened for anti-inflammatory activity in the presence of PMA-induced inflammation, by measuring TNF-α, IL-8 and MCP-1 levels using THP1 monocyte cell system with reference to piroxicam and pioglitazone. It was found that none of the compounds showed any anti-inflammatory activity.
3.5. In vitro Anticancer Activity
- The cytotoxic activities against six cancer cell lines were determined using MTT assay represented in Table 3. All the compounds (1-3) inhibited the growth of different cell lines. The cytotoxic effect of these compounds tested on all the cancer cells are in order of 3-O-methyl Fusarubin > Fusarubin >Javancin. The compound (2 & 3) showed potential cytotoxicity activity against MDA-MB-231 cells with 1.2 and 1.5 µM respectively. The compound (3) showed most cytotoxic effects to MCF7, DU142, MDA-MB-231 and A549 with the IC50 values of 3.4, 2.3, 1.2, 4.1 µM respectively and moderate activity against B16F10 and HeLa cell lines with IC50 values of 8.6 and 10.9 µM. The compound (2), showed good anticancer activity against MDA-MB231, DU142 cell lines with IC50 values of 1.5 and 4.2 µM and moderate activity towards MCF7, HeLa, A549, and B16F10 cell lines with IC50 values of 7.7, 15.6, 16.04 and 10.3 µM respectively. From the past few years, naphthoquinones have been paid special interest due to their anticancer properties. Many quinone groups containing compounds like anthracyclines, daunorubicin, doxorubicin, mitomycin, mitoxantrone and saintopin are currently available drugs in the market for the treatment of solid cancers [30]. Recently, it is reported that the importance of fungal derived naphthoquinones derivatives isolated from different sources showed cytotoxic effect against different cancer cells [23, 11, 31, 32]. The anticancer activity of these naphthoquinones mostly act on topoisomerase-II that ultimately interfere with DNA inhibition and also on the signal transduction–related molecules that might have resulted in the apoptotic cell death [33, 12].
|
4. Conclusions
- From the present study, it is depicted that the compounds 3-O-methyl Fusarubin showed significant antimalarial with lesser-hemolytic properties which ensure it to be safe and can be used as an excellent chemotherapeutic agent for the treatment of life-threatening disease like malaria. On the other hand, 3-O-methyl Fusarubin also showed a potent anticancer agent which can also be used in cancer chemotherapeutics. On the basis of various biological activities of Javanicin, 3-0-Methyl Fusarubin and Fusarubin further studies are required to determine its pharmacological properties both in in vitro and in vivo models before use as therapeutic agents.
ACKNOWLEDGEMENTS
- The authors declare that they have no competing interest. The research was supported by Council of Scientific and Industrial Research, (CSIR), New Delhi fellowship to KPK. Dr. P. Mangala Gowri, Senior scientist CSIR-IICT, Hyderabad and Dr Purna Singh Sijwal, Principal Scientist, CSIR-CCMB for helping in analyzing the chemical structure elucidation part and antimalarial activity respectively.
Supplementary Data
- Materials and methodsSoil sample and Fungal IdentificationThe fungus used in this study was isolated from the soil sample collected from the Nallamala forest region, India and identified as Fusarium sp. USNPF102 based on morphological and molecular data. Briefly, the fungal colony is woolly to cottony with white aerial mycelium and orange to brownish pigment on agar medium. Hyphae are septate and hyaline. Macroconidia are multiple - celled with slightly curved at the pointed ends, Microconidia are one-celled, hyaline and ovoid shape which are the characteristics of the genus Fusarium (Barnett and Hunter 1998).
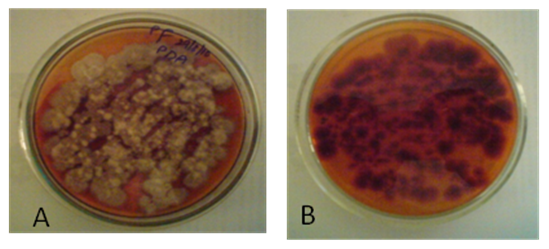 | Figure 1. A. Pure culture of Fusarium sp. USNPF102 on PDA Slant; B. culture on its reverse side |
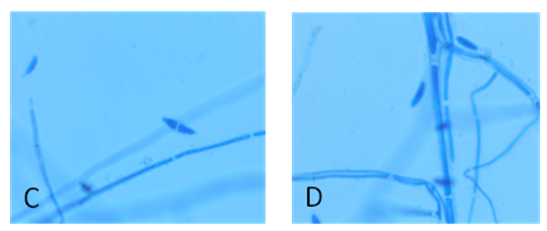 | Figure 2. C & D Microscopic images of Fusarium sp.USNPF102 at 45X and 100X magnification |
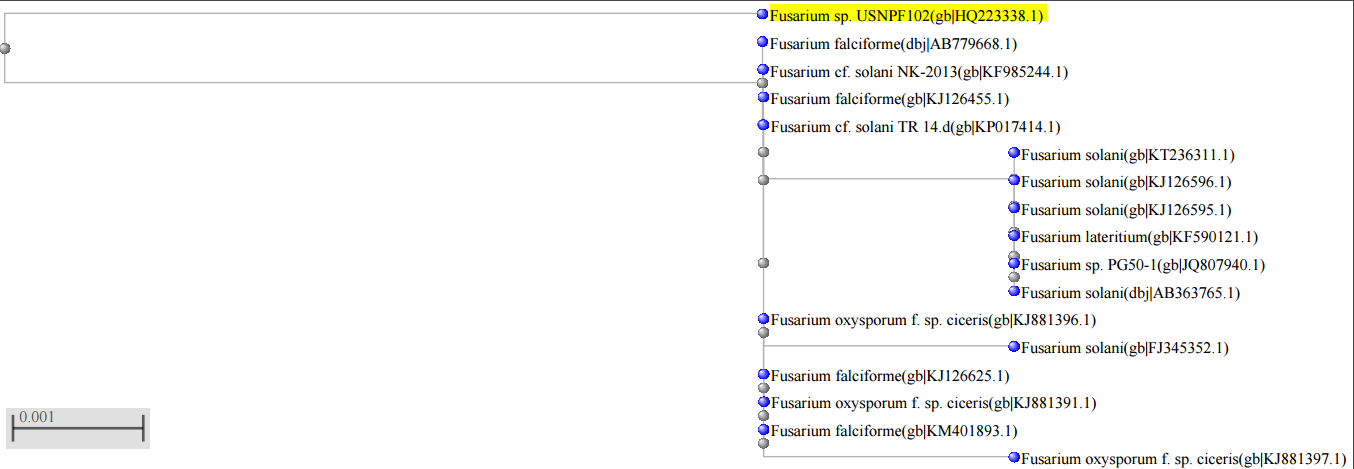 | Figure 3. Phylogenetic tree of Fusarium sp. USNPF102 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML