-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(1): 14-22
doi:10.5923/j.microbiology.20170701.03

Communities of Heterotrophic Bacteria in Gleysoles and Planosoles of Irrigated Rice Fields
Jeremias Pakulski Panizzon1, Harry Luiz Pilz Júnior1, Neiva Knaak2, Renata Cristina Ramos3, Denize Righetto Ziegler3, Lidia Mariana Fiuza2
1PPG Biologia – Universidade do Vale do Rio dos Sinos; Av. Unisinos, São Leopoldo – RS, Brazil
2IRGA – Instituto Rio-Grandense do Arroz; Av. Bonifácio Carvalho Bernardes, Cachoerinha, RS, Brazil
3ITT NUTRIFOR - Universidade do Vale do Rio dos Sinos, Av. Unisinos, São Leopoldo – RS, Brazil
Correspondence to: Neiva Knaak, IRGA – Instituto Rio-Grandense do Arroz; Av. Bonifácio Carvalho Bernardes, Cachoerinha, RS, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work evaluated the effects of physical and chemical variables in the diversity of bacterial communities present in rice field soils in two areas of different regions of southern Brazil. The samples were collected along 2013/14 and 2014/15, in rice producing areas of the regions: Outer Coastal Plain (OCP) and Inner Coastal Plain (ICP), Rio Grande do Sul, Brazil. The results were analyzed using Component Principal Analysis (PCA), Canonical Correspondence Analysis (CCA), Diversity Index, and Analysis of Variance. Were recorded 29 Colony-Forming-Units (CFUs) in both regions and the diversity was similar differentiated only for different phenological phases of plants. Bacterial abundance of colonies corresponding CFUs in OCP was (F1.9 = 7.84 p < 0.05): 18.5% in before sowing; 22.3% in vegetative phase; 22.3% in reproductive phase and 36.9% during seed maturation. Bacterial abundance of colonies in ICP was (F1.9 = 7.03 p < 0.05): 23.9% in before sowing; 21.7% in vegetative phase; 20.7% in reproductive phase and 33.7% during seed maturation. The first two axes of each crop fields generated by CCA in the 2013/14 crop explain 67% of the variation observed and in 2014/15 explain 86.5%. The most important variables for ordination and correlation with the axis were content of organic matter and clay. With an analysis of the entire dataset, the PCA explained 64.84% and a CCA explained 70.7% enter the main two axes, highlighting clay and organic matter. In both types of soils, gleysoles and planosoles, the CFUs of the following communities of heterotrophic bacteria were higher: Bacillus thuringiensis, Bacillus cereus, Lisinibacillus sphericus, Pseudomonas fluorescens, P. putida, Corinebacterium spp.
Keywords: Microorganisms, Rice producing regions, Physicochemical parameters
Cite this paper: Jeremias Pakulski Panizzon, Harry Luiz Pilz Júnior, Neiva Knaak, Renata Cristina Ramos, Denize Righetto Ziegler, Lidia Mariana Fiuza, Communities of Heterotrophic Bacteria in Gleysoles and Planosoles of Irrigated Rice Fields, Journal of Microbiology Research, Vol. 7 No. 1, 2017, pp. 14-22. doi: 10.5923/j.microbiology.20170701.03.
Article Outline
1. Introduction
- Rice (Oryza sativa) is one of the most consumed foods in the world, being one of the most important grains in global economic terms. The rice agro-ecosystems in Brazil produce about 11,000 kg/ha annually, and over 60% of this production is in the state of Rio Grande do Sul [1]. The biodiversity in these agroecosystems refers to all species of plants, animals and microorganisms that interact with the environment [2]. Studies on the factors that influence the distribution, diversity and structure are relatively recent. In-depth study of microbial communities living in aquatic agro-ecosystems is fundamental to a better understanding of what happens in the soil, since these microorganisms play important roles for habitat maintenance [2]. The diversity present in the rhizosphere is different from the rest of the soil because the microorganisms of that region are directly related to plant growth. The bacteria that live there receive plant nutrients as well as antimicrobial agents which are selective and that inhibit certain undesirable microorganisms. A better understanding of environmental factors that influence changes in the diversity of the bacterial communities is very important because they realize functions that are essential for the maintenance of ecosystems [3, 4]. Several studies have analyzed the effects of chemical substances used for fertilization and pest control on bacterial diversity [5] associated with cultivation [6, 7]. These studies mainly analyze the impact of different factors on biodiversity of the bacteria in soil. Other works deal mainly with the relationship of bacteria in soil of rice culture, which are involved in the processing of waste from rice plants [8, 9]. This study aimed to evaluate the diversity of bacterial communities present in the two types of soil: Gleysoles and Planosoles: i) Physical-chemical and microbiological analysis of gleysoles (Outer Coastal Plain) type soil; ii) Physical-chemical and microbiological analysis of planosoles (Inner Coastal Plain) type soil; iii) Comparison between the two soils types studied through statistics elements.
2. Materials and Methods
2.1. Study Area
- The town of Santo Antonio da Patrulha (Outer Coastal Plain -OCP) is bathed by the Sinos River, in the northeastern region of the state of Rio Grande do Sul (RS). It covers an area of 3,820 km2, and flows into the delta of the Jacuí River. In the Sinos Basin, there are thirty-two districts with 975,000 inhabitants, which directly obtain water for consumption and disposal of sewage. Rice fields take water from the same river for irrigation [10]. The city of Charqueadas (Inner Coastal Plain -ICP) is bathed by the Jacuí River. The Jacuí Basin has an area of 71 600 km2, corresponding to 83.5% of the area of the Guaíba River region. The river originates in Jacuí Plateau in the municipalities of Passo Fundo and Marau, and all its drainage area is characterized by intensive use of land for agriculture and livestock [11]. The places analyzed with their geographic coordinates taken from Google (Figure 1) were the districts of Santo Antônio da Patrulha (PCE)- RS: 29° 50' 18'' S and 50° 30' 58'' W and Charqueadas (PCI) - RS: 29° 97' 04.9'' S and 051° 31' 33.2'' W.
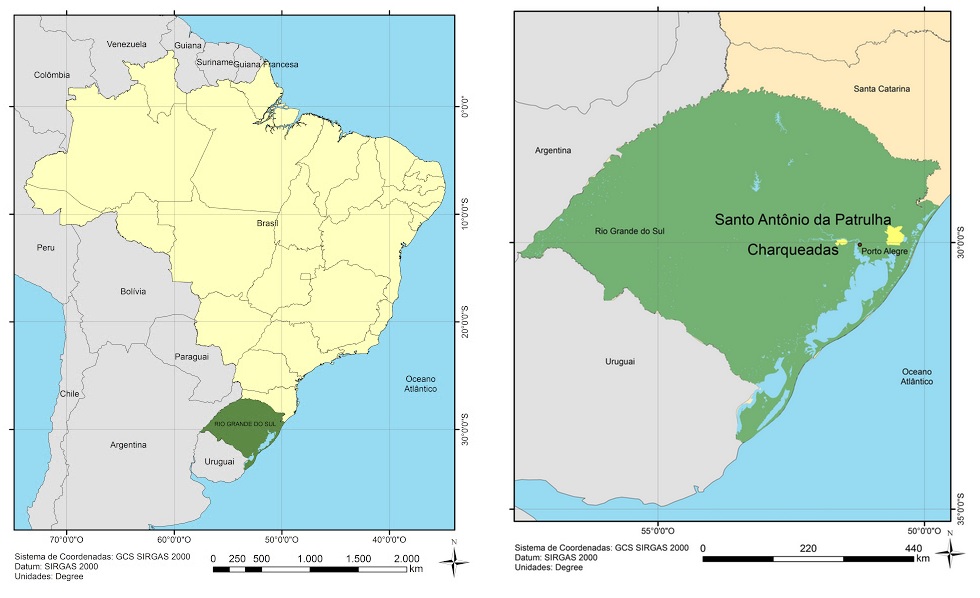 | Figure 1. Location of the areas of Santo Antônio da Patrulha / RS and Charqueadas /RS, Brazil, during the agricultural years of 2013/14 and 2014/15, evaluated in this study |
2.2. Soil Sample
- Data collection took place during both years: 2013/14 and 2014/15. Two rice-growing areas with different soils were chosen. The Outer Coastal Plain (Santo Antônio da Patrulha) has for the most part soil of the Gleysoles type, which has a high rate of organic matter. The Inner Coastal Plain (Charqueadas) has for the most part soil of the Planosoles type, in which its deeper layers are cemented with few nutrients, hindering plant growth. In each farm, soil samples were collected in triplicate. Each sample was made up of sub-samples collected randomly, covering a representative area of the property. The crops were divided into homogeneous areas identified by relief, vegetation, soil type, use and management. They were collected between 10 and 20 sub-samples to form a single sample, crossing a zigzag path at random, covering the entire area. The rocks, roots and debris from the soil surface cultures were eliminated at the collection point. Sampling was done with auger at a depth of 20 cm at each collection point, and the soil was stored in sterile vials. After harvesting, the soil was homogenized and a sample of 500 grams was gathered [12]. After collection, the samples were placed in sterile vials and sent to the Microbiology and Toxicology Laboratory of UNISINOS University.
2.3. Studied Rice Plant
- The sampled cultivar was Puitá INTA-CL in crops of minimum tillage, which are areas that are not heavily manipulated throughout the planting process. Cultivar Puitá INTA-CL is a variety resistant to herbicides, with an average cycle of 125 days, having low height, with an average height of 86 cm which makes it resistant to lodging. Samples were collected into four periods of the crop cycle: one collection after soil before sowing, two collections in the vegetative phase, two collections in the reproductive phase and two during seed maturation, with a total of seven collections in each crop year for each crop [12].
2.4. Diversity
- Bacteria growth initiated from 100 mL water with soil (10g), in which an aliquot of 500 µL was withdrawn in 4.5 mL saline (NaCl 10%) dilution reaching 1.10-3. Then, 100 µL was applied in a Petri dish containing Nutrient Agar [digestible gelatin enzyme (5 g / L), beef extract (3 g / l) and agar (15g / L)] [13], which was incubated in bacteriological incubator at 30°C for 24 hours. The individual colonies grown were counted using a colony counter and the bacterial cells were examined cytologically by differential interference phase microscopy (1,000×). The bacterial spores were separated by pasteurization, and both groups (sporulating and non-sporulating) were identified by cell morphology, physiological and biochemical characteristics, according to international methods of bacterial classification described in Bergey (2010) [14] and the adapted identification key. Gram-negative bacteria were identified by API Biomerieux® 20 E method with subsequent use of APIWEB software for the determination of species.
2.5. Analyzed Parameters
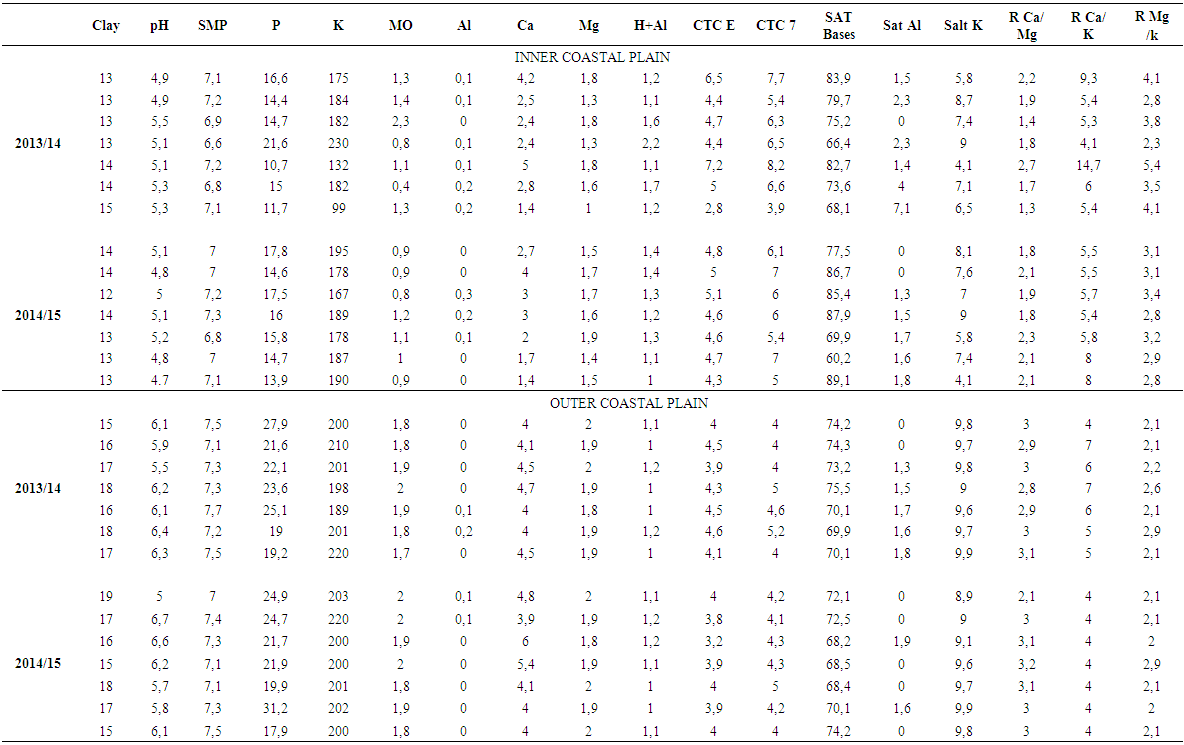
- Physicochemical elements of the soil were analyzed in the EEA-IRGA and the Soil Analysis Laboratory: Clay (%), pH, Index of analysis and correction of acidity - SMP, Phosphorus (mg/L), Potassium (mg/L), Organic Matter - MO (mg/L), Aluminum (mg/L), Calcium (mg L), Magnesium (mg/L), Hydrogen + Aluminum (mg/L), Effective electron exchange capacity – CTC E (mg/L), Electron exchange capacity pH 7 – CTC 7 (mg/L), Saturation (Sat) Bases (%), Sat Al (%) Sat K (%), Relationship (R) Ca/Mg (mg/L), RCa/K (mg/L) e RMg/K (mg/L). The procedures used to estimate the physical and chemical variables were defined by Tedesco et al. (1999) [15].
2.6. Statistical Analysis
- Firstly, to determine the variation of the soil quality, the results were organized into two banks of different data and analyzed separately. The first databank was utilized to evaluate the Gleysoles and the second for planosoles. Analysis of variance (ANOVA - p <0.05) was used to evaluate the differences in the abundance of bacterial colonies in the soil of each field studied, at different periods of plant cultivation. Diversity in the rice fields was analyzed using the Shannon index [16] and Eveness, calculated separately for each town, using the totals for each environmental abundance. The expression values were transformed by log10 (X + 1) to compensate for deviations caused by lower or higher abundance [17]. The influence of physicochemical parameters on the abundance of the main species was quantified by canonical correspondence analysis (CCA) method using the PC-ORD 6.0 software [18]. With the CCA it was possible to produce an analysis of direct ordination gradient, explaining the distribution of species in relation to environmental variables. The meaning of the main axis of the canonical ordination was evaluated by Monte Carlo [17] permutation test. For this analysis it was used species with more than five colonies per point. After the separate analyzes, we did analyzes with the whole database. For the PCA analysis we used a correlation matrix, because the variables are estimated in different units of measurement. Only autovalues greater that 1 were utilized as criteria for extraction of the principal components. The varimax rotation, was used to simplify the expression of a particular sub-space in terms of just a few major items each, the actual coordinate system is unchanged, as to facilitate the interpretation of the data generated by the principal component analysis. We used PCA in the PAST 3 program.
3. Results
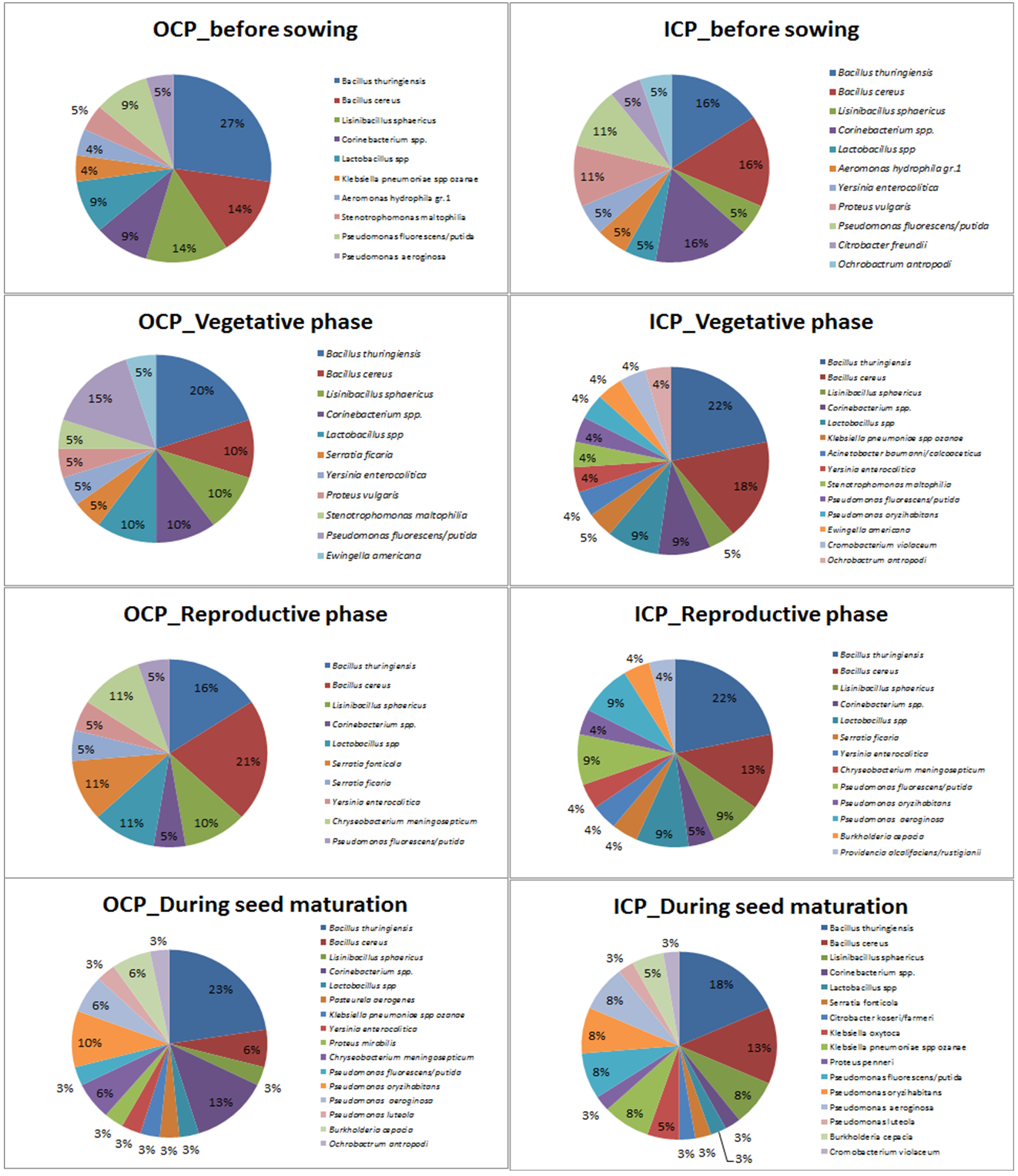
- In the analysis of the results it was recorded a total of 29 species; of these, 27 were found in Outer Coastal Plain-OCP and 22 in Inner Coastal Plain-ICP in different periods of crop cultivation. Abundance of bacterial colonies in Outer Coastal Plain (F1,9 = 7.84, p <0.05) was 18.5% in the pre-planting period, 22.3% in the vegetative phase, 22.3% in the reproductive phase and 36.9% during maturity. The Shannon index estimated diversity was more pronounced at maturity (M = 3,901) compared to the values obtained in the soil of other phases (M = 2.97). Abundance of bacterial colonies in Inner Coastal Plain (F1,9= 7.03, p <0.05) was 23.9% in the pre-planting period, 21.7% in the vegetative phase, 20.7% in the reproductive phase and 33.7% during maturity. The Shannon index estimated diversity was more pronounced at maturity (M= 3,801) compared to the values obtained in the soil of other phases (M= 2.46). The Evenness index indicates that the distribution of species is similar in both locations during maturity (E= 0.988 and 0.967). Figure 2 identifies the microorganisms found in the soil samples in the areas of rice cultivation in the studied cities.
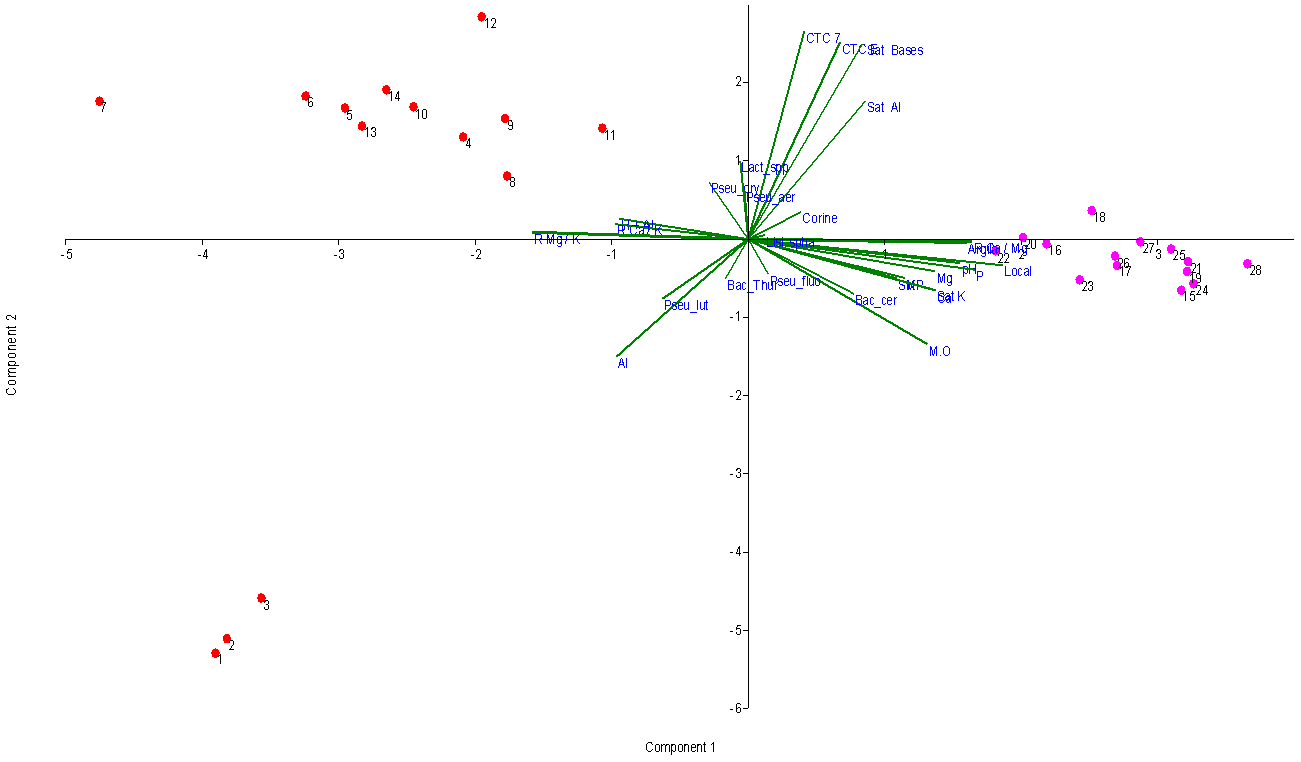 | Figure 3. Biplot graph showing the separation between Inner Coastal Plain and Outer Coastal Plain and principal components |
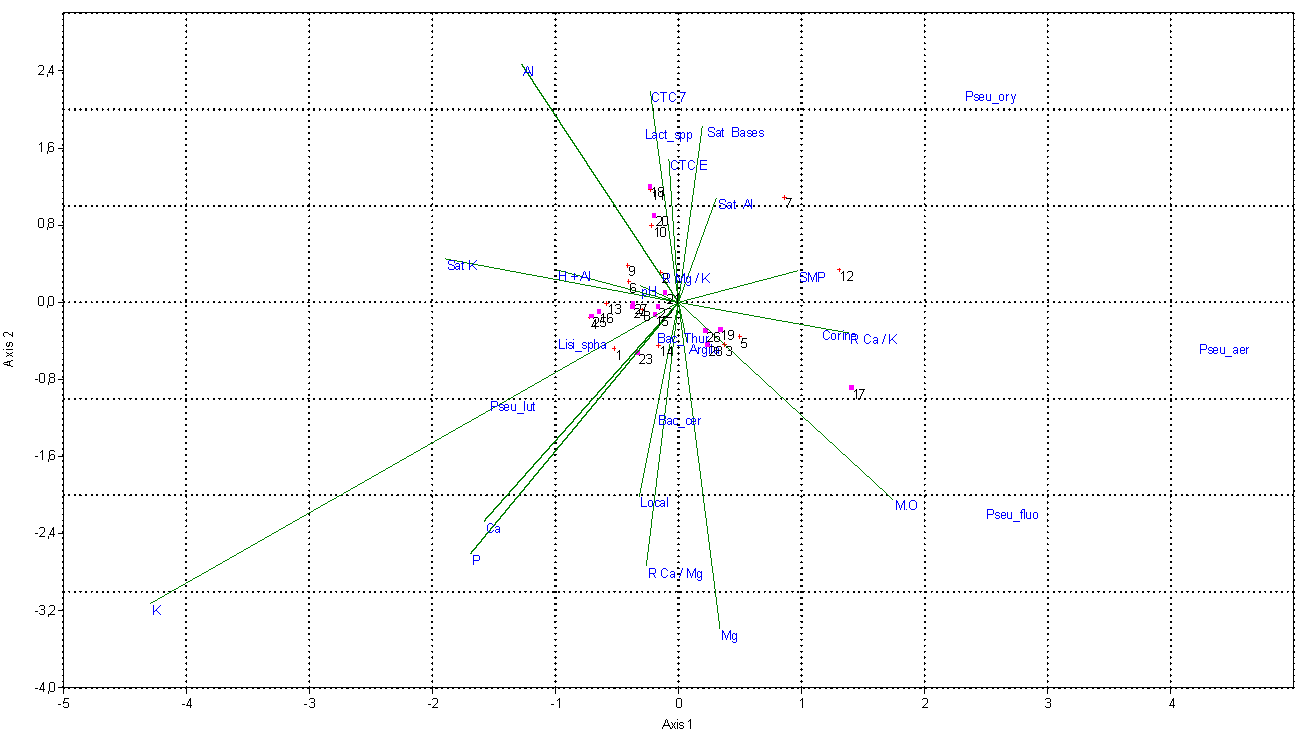 | Figure 4. Canonical correspondence analysis (CCA) of bacterial communities obtained from soil of rice crops in Outer Coastal Plain and Inner Coastal Plain, RS / Brazil |
4. Discussion
- The bacterial communities found in agricultural soils of Outer Coastal Plain and Inner Coastal Plain / RS are composed of several species, but with low abundance - such as Bacillus cereus, Bacillus thuringiensis, Pseudomonas fluorescens and Lactobacillus spp. Other authors also found similar results evaluating the bacterial diversity in soil [9-20]. In an agroecosystem, the diversity variation throughout the seasons of the year is still not well understood, as in every season it seems to occur dominant microbial community accompanied by other less abundant that often are below the detection level of the current evaluation methods [21]. The species B. thuringiensis was the most frequent in soils of rice cultivation. These bacteria can act as entomopathogens in the biological control of pests, especially in rice culture. This species was also prevalent in the work of Panizzon et al. (2012) [10] in water samples from rice cultivation areas. Bacteria from the genus Pseudomonas are a major group from soil, especially because of their ability to act as endophytes contributing significantly to the development of plants [22-23]. Microorganisms have great importance in biochemical and geochemical cycles and have a great chemical and molecular arsenal. In the context of rice cultivation, bacterial abundance can be considered by its wide variety of microhabitats, caused by constant irrigation, resulting in complex bacterial communities [24]. In a similar study, Panizzon et al. (2012) [10] evaluated the effects of physical and chemical variables on the diversity of bacterial communities present in the water used to irrigate rice fields, the water from the central part of the crop and the water drained back into the river. They found that the abundance of bacterial colonies was higher in the irrigation water than in the rice field or the drainage. In addition, the bacterial community in the water samples from rice growing areas in the Sinos River Basin, RS, Brazil, contains several species, but few are abundant, such as the genus Bacillus, Lactobacillus and Pseudomonas, confirming the results found in this study in rice culture soil (Table 1). Cortés-Sánchez et al. (2015) [25] report that cosmopolitan microorganisms isolated from soil, water and vegetables such as bacteria of the Enterobacteriaceae family can also be found in the digestive tract of animals and humans, although it is also possible to find them in transient or normal microbiota. Among them include: Klebsiella spp., Enterobacter spp., Serratia spp., Citrobacter spp., Yersinia spp., Proteus spp., Providencia spp., Shigella spp., Ewingella ssp., Erwinia spp. and Pantoea spp. They can be pathogenic or opportunistic, occasionally forming components with physicochemical and biological properties beneficial to humans and the environment in which they operate. According CCA analysis, the clay is associated with bacterial diversity. This element is essential for the production of annual crops and its low availability impairs productivity. The impact of nitrogen and clay in bacterial communities in soil is difficult to analyze and restrains the visualization of patterns, although it is associated in some studies with a reduction in biomass [26-28]. Microbial communities are particularly affected by the management and impact in the ground. Agricultural practices such as soil alterations during preparation and irrigation can modify bacterial communities. Being the organic matter one of the main elements for good survival of microorganisms [29]. The bacteria found in soil are highly diversified. In hot soils, for example, there is the presence of thermophilic microorganisms and microbial population changes very quickly as the available nutrients are modified [30]. The amount of organic matter significantly influenced the microbial growth (Table 3). Usually the rivers that irrigate the rice fields receive large amounts of sewage, which can contribute with antimicrobial substances that help forming the bacterial community in the studied region. Heavy metals also present in the sources supplying the crops may inhibit growth of some bacteria, such as the case of mercury. However, many Pseudomonas survive in environments and thus convert the mercury into methylated mercury, which is volatile and escapes to the atmosphere [31, 32]. Thus, a soil with high organic matter content tends to maintain the microbial population more stable throughout the year, probably due to the richness of ecological niches, through the heterogeneity of carbon sources [33]. The CCA analysis shows an explanation of 67% for the year 2013/14 and 86.5% for the year 2014/2015. Such variations are directly linked to the water system and climate of the region, the structure and soil management, and the content and quality of accumulated plant residues [34]. In this case, the small difference found in relation to the two agricultural years evaluated may be related to high rainfall during the agricultural year of 2013/14. However, the differences are shown in only a few species which may be present or absent at any place to a greater or lesser degree. If we remove those species, the other diversity values of the two areas are similar. The CCA analysis (Fig. 3) shows an explanation of 77,07% for the two years together. The PCA analysis explanation of 64,84% (Fig.4). There are two different soils, so the physical-chemical variables are different. The Outer Coastal Plain has a more nutritive soil than Inner Coastal Plain. This situation directly affects the development of the plant. But, with the data obtained in this study we concluded that the bacterial diversity is similar in the two areas studied, regardless of soil type. The difference is for bacteria in each soil. According to Reche et al. (2016), phosphorus, potassium and calcium are the nutrients that have most appeared to be influencing the development of the rice plant. The authors presented a reduction of potassium in the maturation period, similar to what happened in our research. It is known that pH, phosphorus and potassium help the plant to develop.
5. Conclusions
- The bacterial diversity was influenced by the parameters Clay and organic matter. The findings are a contribution to the study of factors that influence the diversity of heterotrophic bacterial communities in rice fields. Microorganisms are essential in the natural processes responsible for the quality of soil and, therefore, knowledge on microbial diversity of the system and the factors influencing them are of considerable importance.
ACKNOWLEDGEMENTS
- We thank God. We also thank our families and friends. We thank CNPq, FAPERGS, Irga, UNISINOS and NUTRIFOR.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML