-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(1): 8-13
doi:10.5923/j.microbiology.20170701.02

Microbiological Study of the Soil in Hail Industrial Zone, Kingdom of Saudi Arabia
Abdalla S. Al-Shammary , Abdel Moneim E. Sulieman , Adil A. Abdelmageed , Vajid N. Veettil
Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
Correspondence to: Abdel Moneim E. Sulieman , Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Soil samples were collected from two depths of seven different locations within the Industrial zone which is located at Eastern side of Hail city. The total bacterial and fungal (yeast and moulds) counts of the soil samples were estimated using standard spread plate technique. The bacterial and fungal isolates were identified by their cultural, morphological and biochemical characteristics using established procedures. Bacterial counts were in the order of 104-107 cfu/g of soil, while yeasts and mould counts were in the order of 103-106 cfu/g of soil. The highest bacterial count was observed in sample 7-1, while the lowest value was seen in sample 2-2. Fungi counts were high in samples 7-1 and 7-2 and lower in samples 2-2 and 4-2. All soil samples taken from the soil surface (A) contained higher microbial counts compared to those collected from soil depth (B). Similar bacterial and fungal genera were identified in the different sampling sites in course of the present study, but their occurrences and levels of predominance were different. Bacillus spp dominated the bacterial isolates while Aspergillus spp was the most overwhelming fungus over the diverse sampling sites. Bacterial and yeasts and moulds abundance were common of an environment with high species richness and functional diversity.
Keywords: Yeasts and moulds, Bacillus, Aspergillus, Biochemical characteristics
Cite this paper: Abdalla S. Al-Shammary , Abdel Moneim E. Sulieman , Adil A. Abdelmageed , Vajid N. Veettil , Microbiological Study of the Soil in Hail Industrial Zone, Kingdom of Saudi Arabia, Journal of Microbiology Research, Vol. 7 No. 1, 2017, pp. 8-13. doi: 10.5923/j.microbiology.20170701.02.
Article Outline
1. Introduction
- Soil is a natural body consisting of various horizons of mineral constituents, each with different proportions and has some distinctive features. Besides minerals, soil is included soil organic matter, water and air. The arrangement and extent of these parts significantly impact soil physical properties like structure and porosity. Soil bacteria and fungi play a vital role in different biogeochemical cycles and are in charge of the cycling of natural mixes [1].Soil is a fascinating medium for developing microorganisms, as it contains different supplements that the organisms require for their digestion system. Unfortunately, nutrients are not generally promptly accessible [2]. However, it is one of the richest reservoirs of microorganisms, i.e. 1 gram of agricultural soil may contain even several billion colony forming units of microorganisms belonging to thousands of different species, and even though microorganisms constitute less than 0.5% of the soil mass, they have a major impact on soil properties and processes. Destruction of the soil microbiota through bungle or natural contamination causes decline or even death of the above ground plant and animal populations. The most characteristic feature of microbial habitats is the great micro-spatial variability of environmental parameters, like temperature or nutrient availability. Many basic requirements of heterogeneous microorganisms are satisfied by various soil microhabitats [3]. Many researchers emphasize the influence of soil structure and spatial isolation on microbial diversity and community structure [4]. Some studies indicate that the soil particle size affects the diversity of microorganisms and community structure to a greater extent than other factors such as bulk pH and the type or amount of available organic compounds [5]. Other investigations demonstrate that the type and measure of available organic substrates firmly influence the abundance of microbial groups and their functional diversity in soils [6].The microorganisms pose a significant threat towards the soil environment and the rapid industrialization results in increasing problems of environmental pollution and soil contamination may endanger human health through skin contact and inhalation of dust particles. Studies on the microbiology of the soil of the industrial zone in Hail are lacking, therefore the present study was initiated to study the diverse population of bacteria and yeasts and moulds in soil of Hail industrial zone during the period August 2016-January 2017. Determination of the microbiology of soil will give a view to understand the microbial flora that are found in the different top soils of the sampling area. The findings of this study might reveal insight into the microbial assets show in this group in lieu of planning ways and implies by which their possibilities might be ideally bridled.
2. Materials and Methods
2.1. Collection of Soil Samples
- Soil samples were collected from two horizon levels A (Soil surface), and B (20 centimeter depth from 7 different sites in the industrial zone, Hail city. The soil samples in these sites may experience intense contamination with oils, detergents and traffic conditions. The selection of the sampling sites is mainly based on the approach that sampling must be carried out wherever possible that may assure high rates of polluted soils. The samples were transferred to an air-tight pre-sterilized polyethylene bag before transportation to the laboratory, numbered, and labeled with date and site of collection. When samples could not process immediately, they were stored at 4°C for no longer than 18 to 24 h.
2.2. Microbiological Analyses
- For determination of microbiological characteristics of the soil samples, 1 g of soil was added into nutrient agar medium and incubated at 170 rpm at 30°C in an orbital shaker. One ml of the soil suspension was then serially diluted (ten-fold) and used in the estimation of aerobic heterotrophic bacterial and yeast and moulds counts by standard spread-plate dilution method described by Seeley and VanDemark [7] in triplicate. Visible discrete colonies in incubated plates were counted and expressed as colony forming units per gram (cfu/g) of soil samples.The isolates obtained were purified by repeated sub-culturing on fresh agar medium and incubated under normal condition for growth. Pure colonies isolated were inoculated on agar slants using MacConkey bottles to serve as stock cultures, incubated at 37°C and were stored in the refrigerator at 6°C ± 2°C for future research. For identification of bacteria species, the 10–2 dilution were placed in Bushnell Haas medium containing 15.0 g/L of agar. Inoculated plates were purified repeatedly by sub culturing. Pure culture was stored in nutrient agar slants at 4°C. The presumptive identification of bacterial species was done using morphological tests as well as biochemical tests using APi identification kits according to manufacturer’s instructions.For identification of yeasts and moulds species, Potato dextrose agar to which 0.05% (w/v) chloramphenicol had been added (to inhibit bacteria growth) was used for yeasts and moulds isolation, and incubation was at ambient temperature for seven days. Pure isolates of representative communities were maintained on agar slant at 4°C. Identification of isolates was based on cultural, microscopic, and biochemical.
2.3. Total Coliforms, Faecal Coliforms and Escherichiacoli
- A reference method based on most probable number (MPN) was used to estimate total coliforms, faecal coliforms and Escherichia coli. Pre-enrichment was done in LST broth (37 _C for 48 h) and confirmation tests were done in BGLB broth for total coliforms (37 _C for 48 h) and in EC broth for faecal coliforms (44 _C for 24 h). Escherichia coli were confirmed by the testing of indole production. The expression of results is in cfu/ g.
2.4. Statistical Analysis
- The data was statistically analyzed using one-way Analysis of Variance (ANOVA) and Pearson chi–square statistics at probability of P<0.05 and P<0.01. Relationships were tested for using the Pearson correlation index at the same probability. All statistical analysis was performed using SPSS 11.0 software.
3. Results and Discussion
- The diversity of soil microorganisms includes various levels of biological organization. It includes genetic variability among taxa (species), number (richness), relative abundance (evenness) of taxa and functional groups within communities [4]. The overall biodiversity of soil microflora comprises bacteria, fungi, actinomycetes and photosynthetic microorganisms [2].
3.1. The Total Bacterial Counts
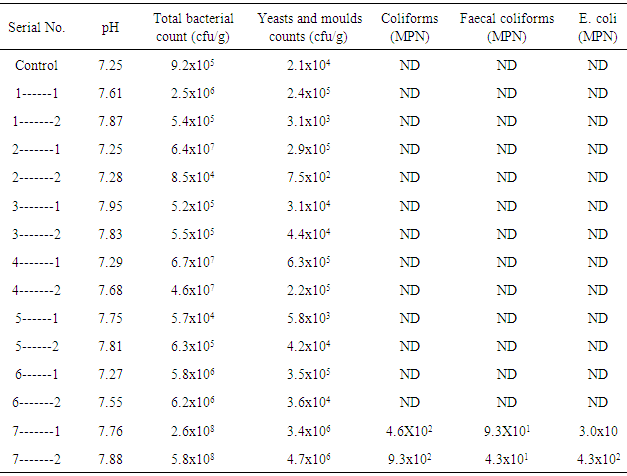
- The results of the total bacterial counts (TBC) of the various soil samples taken from various soil depths of Hail industrial zone are presented in Table (1). The mean of soil samples ranged from 5.8x108 (cfu/g) to 5.7x104 (cfu/g). Although there were differences in the averages total bacterial counts of the different sampling sites, these differences were not statistically significant. However, highest counts were observed in samples 7-1 and 7-2, and lowest count was observed in 5-1 compared to that of the control samples which was 9.2x105cfu/g. Generally, the bacterial counts in A bedon and decreased in B bedon. This could be attributed the reduction of organic matter in lower levels of soils compared to that at soil surface. In addition, the sampling sites from which samples 7-1 and 7-2 have been located in Aledari valley which is exposed to the rain and washing neighbors.
|
3.2. Total Yeasts and Moulds Counts
- The mean total yeasts and moulds of soil samples ranged from 3.1x103 cfu/g of soil and 4.7x106 cfu/g of soil (Table 1). As for bacterial counts, the highest counts were observed in samples 7-1 and 7-2 which have been taken from located Aledari valley, and the lowest counts were observed in sample 1-2 compared to that of the control sample which was 2.1x104 cfu/g. These values fell within the range reported by other worker [12]. Differences in the average total fungal counts of the sampling locations were not statistically significant. In addition, the total counts of fungi (yeasts and moulds) decreased with an increased soil depth. Therefore, all samples taken from the soil surface contained lower fungi counts.As expect, the total bacterial counts were generally higher than those of yeasts and moulds, irrespective of sampling sites. The predominance of bacteria over fungi observed all through the inspecting time has been accounted for by other researchers [13, 8].Microbial biomass can reflect soil quality. Bacteria and fungi, among soil organisms, effectively take part in natural matter disintegration freeing synthetic supplements and facilitating plant development. These microorganism numbers vary in and between different soil types and conditions, with bacteria being the most numerous [14].
3.3. Total Coliforms, Faecal Coliforms and Escherichia coli
- The results of coliforms test (Table 1) indicted all samples were devoid of coliforms bacteria with exception to samples 7-1 and 7-2 which contained high numbers of coliforms, faecal coliforms and E.coli. These samples as mentioned before had been collected from Aledareh valley which is exposed to the rain and washing neighbors towards the valley which raining water might be leached. It has been demonstrated that a high number of viable bacterial counts and high organic matter could signify a more diverse groups of bacteria such as coliforms, faecal coliforms and Escherichia coli [15, 16].Coliform bacteria originate as life forms in soil or vegetation, furthermore, in the intestinal tract of warm-blooded animals (Faecal coliform). This group of bacteria has for some time been an indicator of water contamination and conceivable nearness of intestinal parasites and pathogen. Coliform bacteria are moderately easy to distinguish, are available in much bigger numbers than more risky pathogens, and respond to the regular habitat and treatment forms comparatively to pathogens. By watching coliforms, the expansion or reduction of numerous pathogenic bacteria can be assessed.
3.4. Identification of Bacteria Genera in Soil Samples
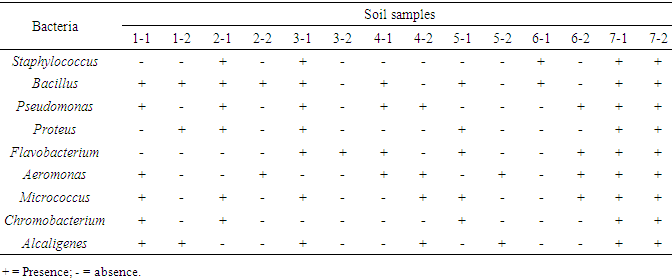
- A total of ten bacterial genera were identified throughout the soil sampling sites, with Bacillus genera being the most dominant. The least genera were Staphylococcus and Chromobacterium as indicated in Table (2). Other bacterial genera included: Pseudomonas, Proteus, Flavobacterium, Aeromonas, Micrococcus and Alcaligenes. Members of the genera Bacillus and Clostridium have long been considered to be common members of the soil bacterial community, members of this group must be considered to be relatively minor components of soil bacterial communities [17].
|
3.5. Identification of Fungal Genera in Soil Samples
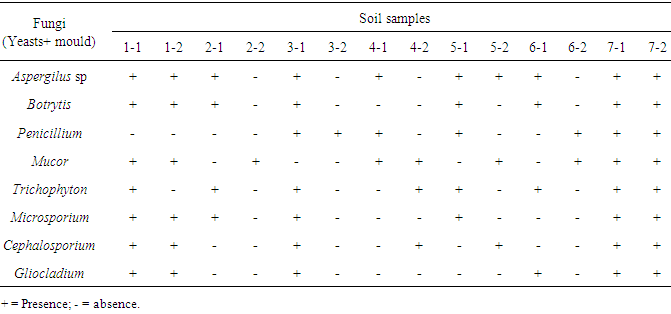
- Throughout the different sampling sites a total of ten fungi were identified with Aspergillus sp as the most dominant and Gliocladium sp being the least dominant as indicated in Table (3). Other fungi included: Botrytis, Penicillium, Mucor, Microsporium, Microsporium and Trichophyton.
|
4. Conclusions
- The outcome of this study has been able to show that diverse species of bacteria and fungi were isolated from Hail industrial zone, Kingdom of Saudi Arabia. Although samples were collected from different sites, the species of bacteria and yeasts and moulds isolated were generally similar, though occurring at different sites during the study period. The abundance of bacteria and yeasts and moulds in this study were typical of environment with high species richness and functional diversity. Despite the fact that the consequences of this study would not be thought to be comprehensive, as it was done within the limited facilities available in the laboratory, a knowledge into the population dynamics and distribution of cultural bacteria, yeasts and moulds diversity has been explained. It would require more cutting edge innovation (cores corrosive tests to obtain such detailed overview of microbial diversity. This ought to be a subject of augmentation of this examination in future.
ACKNOWLEDGEMENTS
- The authors express their sincere gratitude to the Deanship of Scientific Research of University of Hail, Saudi Arabia for financing this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML