-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2017; 7(1): 1-7
doi:10.5923/j.microbiology.20170701.01

Ants as Vectors of Bacteria in Hospital Environments
Bruna Rafaela Machado Oliveira1, Luciano Ferreira de Sousa1, Raquel Chalá Soares2, Thiago César Nascimento3, Marcelo Silva Madureira1, Jorge Luiz Fortuna1
1Universidade do Estado da Bahia, UNEB, Campus X, Teixeira de Freitas, Bahia, Brazil
2Commission for the Control of Hospital-Acquired Infections, Hospital Municipal, Teixeira de Freitas, Bahia, Brazil
3Universidade Federal de Juiz de Fora, UFJF, Juiz de Fora, Brazil
Correspondence to: Jorge Luiz Fortuna, Universidade do Estado da Bahia, UNEB, Campus X, Teixeira de Freitas, Bahia, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Ants in hospital environments may carry pathogenic microorganisms that cause hospital-acquired infections (HAIs), developing resistance to antimicrobials and therefore representing a public health hazard. HAIs increase mortality rates, as a constant threat in the spread of multi-resistant bacteria. This study isolated and identified pathogenic bacteria in ants captured in a hospital in Brazil. We also identified the main genera of ants in these environments. Ants were attracted using protein and carbohydrate traps. A group of five ants was considered a sample. Microbiological analysis included the isolation of Escherichia coli and other enterobacteria and staphylococci. Susceptibility was tested against vancomycin, cefoxitin, oxacillin, and chloramphenicol. The results of resistance to chloramphenicol assays revealed the presence of resistant Enterobacteriaceae (E. coli), resistant isolates of Citrobacter feundii, Enterobacter spp., Klebsiella spp., and Klebsiella oxytoca, and susceptible Hafnia spp., and Yersinia enterocolitica isolates. For vancomycin, oxacillin, and cefoxitin, the bacteria included resistant and sensitive coagulase-negative Staphylococcus spp. and coagulase-positive S. aureus CP. The ant genera identified were Pheidole, Crematogaster, Linepithema, and the species Tapinoma melanocephalum. Ants in hospital environments may carry significant numbers of bacteria resistant to the antimicrobials used. This highlights the risk these bacteria pose in such settings, especially to intensive care patients, who usually are immunodepleted.
Keywords: Antimicrobial, Hospital-acquired infection, Resistant bacteria
Cite this paper: Bruna Rafaela Machado Oliveira, Luciano Ferreira de Sousa, Raquel Chalá Soares, Thiago César Nascimento, Marcelo Silva Madureira, Jorge Luiz Fortuna, Ants as Vectors of Bacteria in Hospital Environments, Journal of Microbiology Research, Vol. 7 No. 1, 2017, pp. 1-7. doi: 10.5923/j.microbiology.20170701.01.
Article Outline
1. Introduction
- Urbanization processes pose issues such as inordinate population growth, water contamination, and the difficulty to implement sanitary control measures. Such scenario promotes the dissemination of arthropods and therefore of the diseases they may carry. For this reason, these animals pose some of the most important hazards to quality of life, standing as a growing concern [1-5]. More specifically, the presence of ants in hospital environments is a serious health problem. Several ant species act as mechanical vectors to pathogenic microorganisms, which points to the importance of research on the transport of pathogens associated with ants in the development of measures to reduce the prevalence of hospital-acquired infections (HAIs), decrease associated mortality, and optimize healthcare budgets.Like other social arthropods, ants are attracted by foods or even medical drugs, especially those containing sugar. Ants have impressive mobility features, reaching speeds of 3 cm/s. This advantage grants these insects access to several environments in an hospital, carrying pathogenic microorganisms that pose potential threats and may be associated with HAIs [6].Importantly, the social organization of ants is another factor that induces workers to roam around several hospital environments. In this process these insects may acquire bacteria in potentially contaminated environments like bathrooms, reception desks, and wards, at which point they begin to carry these microorganisms to restricted access settings, such as sterilization rooms, laboratories, nurseries, and operating theatres. The presence of pathogenic bacteria in the tegument of worker ants foraging in essentially any hospital environment qualifies these insects as potentially important agents in the spread of infectious diseases in healthcare facilities [5].HAIs include any infection a patient develops after admission that manifests during this period or even after discharge, as long as the disease may be associated with the inpatient treatment period or medical procedures carried out then [7]. According to a HAI control survey carried out by Brazil’s Sanitary Surveillance Agency (ANVISA), the rate of these infections in the country stands at 9.0% in the country, though in some healthcare organizations this value has been found to be much higher, reaching impressive 88.23% [8].In view of the above, healthcare staff has to be aware of the need for controlling and mapping these insects’ habits as a means to control HAIs [9], whose main consequences are increased morbidity and mortality rates, and higher costs associated with longer inpatient treatments. Moreover, HAIs stand as a constant threat in the spread of multi-resistant bacteria [10]. This highlights the need for studies on this topic as a tool in the surveillance of infestation hotspots and the detection of resulting microbiological contamination. Such research is also important in the design of more efficacious insect control methods, which indirectly help reduce HAI prevalence.Aiming to contribute to the knowledge about ants in urban environments, especially hospitals, the present study identified the pathogenic bacteria (enterobacteria and staphylococci) isolated from ants captured in hospital settings, and tested the resistance of these pathogens to selected antibiotics. We also identified the genera of these ants, and discuss the issues associated with their presence in healthcare environments as a key factor in the development of ant control strategies.
2. Materials and Methods
2.1. Collection of Ants
- Ants were collected in 11 different environments in the Municipal Hospital of Teixeira de Freitas, state of Bahia, Brazil, namely: (i) the intensive care unit (ICU), (ii) the semi-intensive care unit, (iii) food preparation facilities, (iv) the social service office, (v) the emergency room, (vi) the operating theatre, (vii) a nurse station, (viii) ward 107 (medical care), (ix) ward 202 (surgery), (x) ward 208 (orthopaedics), and (xi) the food pantry.Collections took place twice a day, in the morning and early evening in December 2014 and March, May, and August 2015, representing eight collection efforts. In total, 256 protein and carbohydrate traps were used, 128 of each bait. Honey and wiener sausages were used as carbohydrate and protein baits, respectively. Traps were left in each environment for 40 min. Ants were carefully picked with sterilized tweezers and placed in test tubes containing Brain Heart Infusion (BHI) broth. Five ants were placed in each tube, which was labelled identifying the hospital environment of origin (Figure 1). The operator wore sterilized gloves throughout the collection effort [11].
2.2. Microbiological Analyses
- The tubes containing ants in BHI broth were incubated at 35°C for 24 h. Contents with evidence of bacterial growth were seeded on Petri dishes with specific culture media (MacConkey Agar, MCA, for Escherichia coli and other enterobacteria; Mannitol Salt Agar, MSA, for Staphylococcus spp.). Dishes were incubated at 35°C for 24 h [5, 11-13].Typical E. coli colonies and those of other enterobacteria were analysed according to biochemical assays like indole, methyl red, Voges–Proskauer, and citrate (IMViC) to identify and confirm bacteria to species level. Typical Staphylococcus spp. colonies were submitted to the catalase and coagulase assay and to a novobiocin-resistance assay to differentiate Staphylococcus epidermidis and Staphylococcus saprophyticus.
2.3. Resistance to Antimicrobials
- The species identified were submitted to antimicrobial resistance assays according to the disk diffusion method [15, 16].Colonies of each isolate were used to prepare one bacterial suspension with turbidity adjusted to 0.5 MacFarland. Seeding was carried out in Mueller-Hinton Agar (MHA). Oxacillin, cefoxitin, and vancomycin disks were used to evaluate resistance of the isolates of coagulase-positive (CP) Staphylococcus aureus, coagulase-negative (CN) Staphylococcus, S. saprophyticus, and S. epidermidis. One chloramphenicol disk was used to test E. coli and other enterobacteria.
2.4. Identification of Ants
- Ants were characterized to genus level using identification keys in Baccaro et al. [17]. Ant species were identified using the keys available in specialized websites [18].
3. Results and Discussion
- Of the 256 traps laid, 12 (4.7%) attracted ants (eight of which, or 66.7%, with protein and four, or 33.3%, with carbohydrate baits). In total, 60 individuals were captured. Considering all the eight collection efforts, we caught ants only in three (37.5%), namely in the afternoon in December 2014, in the morning in March 2015, and in the afternoon in August 2015.The low number of individuals collected may be explained in view of the fact that half the traps were laid in autumn and winter. A similar finding was observed by Couceiro [19], who collected ants in spring and summer, while only 44 were captured in autumn and winter. Also, the hospital surveyed in the present study had gone through a series of pest control procedures, which prevented more frequent collection efforts. However, insect control was carried out with sulfluramid gel, a slow-release pest control agent, so we managed to collect ants in the environments cited before they actually reached nests.Of the 11 environments surveyed, ants were captured in seven (63.6%), namely the ICU, semi-intensive unit, food preparation facilities, emergency room, ward 107 (medical care), ward 202 (surgery), and ward 208 (orthopaedics).The highest number of ants was captured in the evening. Collections were successful in both morning and evening in ward 107 (medical care) and ward 202 (surgery) (Table 1).
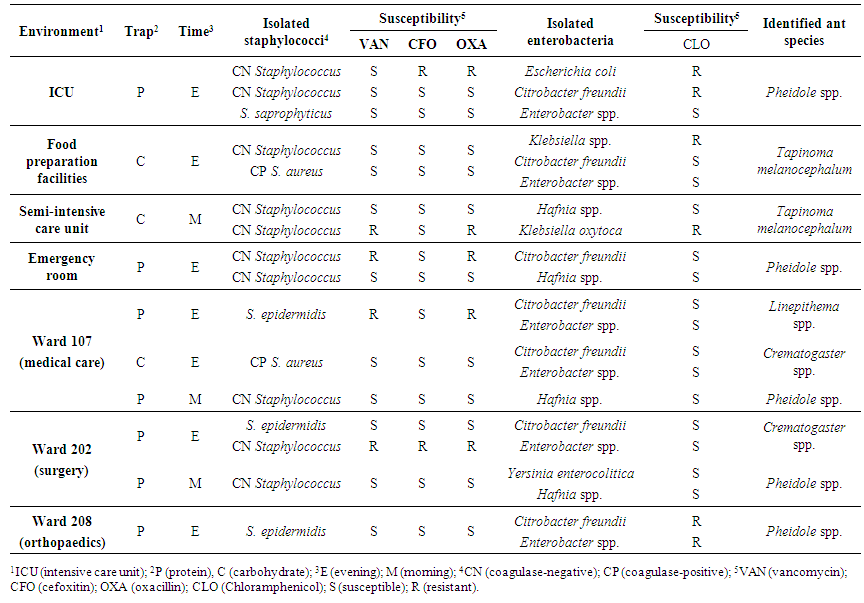 | Table 1. Pathogenic bacteria isolated and identified from ants captured in hospital environments using protein and carbohydrate traps. Susceptibility of strains to selected antibiotics is shown |
4. Conclusions
- The results show that the ants captured are possibly working as carriers of pathogenic bacteria. Transmission may take place directly, when ants crawl up a patient’s skin, or indirectly, when they run on medical devices. Besides the fact that these ants carry a significant number of clinically important bacteria in hospital settings, another relevant finding was the resistance to selected antimicrobials, which increases the risk of HAIs, especially in ICU inpatients who, in most cases, are immunodepleted.The great importance of bacteria carried by ants in hospital environments lies in the resistance to antimicrobials they develop, highlighting the need for increased awareness in healthcare organizations as to the adoption of strict prevention measures. Such initiatives may be as simple as washing hands properly and as complex as devising sensible courses of antimicrobials to inpatients or conceiving efficient pest control programmes.Despite the confirmation that ants carry microorganisms, our results have not afforded to clarify the precise role these insects have in HAIs. Further studies should be conducted to assess the risk of infection in hospital settings potentially colonized by ants.
ACKNOWLEDGEMENTS
- The authors thank Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) for the grants given, and to Comissão de Controle de Infecção Hospitalar (CCIH), Hospital Municipal de Teixeira de Freitas, state of Bahia, Brazil, for allowing us to collect ant samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML