-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(5): 93-102
doi:10.5923/j.microbiology.20160605.01

Isolation and Identification of Cholesterol Lowering Probiotic Bacteria from Palm Wine (Raffia mambillensis)
Eurydice Flore Tiepma Ngongang 1, 2, Bernard Tiencheu 1, 2, Bertrand Tatsinkou Fossi 3, Aduni Ufuan Achidi 1, Dzelafen Marcel Shiynyuy 1, Hilaire Macaire Womeni 2, Zambou Ngoufack François 2
1Department of Biochemistry and Molecular Biology, Faculty of Science, University of Buea, Buea, Cameroon
2Department of Biochemistry, Faculty of Science University of Dschang, Dschang, Cameroon
3Department of Microbiology and Parasitology, Faculty of Science, University of Buea, Buea, Cameroon
Correspondence to: Bernard Tiencheu , Department of Biochemistry and Molecular Biology, Faculty of Science, University of Buea, Buea, Cameroon.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of this study was to isolate and identify potential lactic acid bacteria from palm raffia wine and to evaluate in vivo the effect of isolated probiotic bacteria on serum lipids and some enzymes activities in wistar albino rats. Lactic acid bacteria were isolated on MRS agar using pour plate method. The catalase negative and Gram positive isolates were selected as presumptive lactic acid bacteria. They were also assessed for invitro cholesterol assimilation from culture media, acid tolerance and bile salt tolerance. Selected isolates were biochemically characterized using the API 50 CHL BioMerieux kit. In-vivo cholesterol lowing effect was studied on hyperlipidemic induced wistar rats randomly assigned into three groups. The experiment was carried out for four weeks by oral gavages; dose level 108-1010 CFU/ ml. The administered volume of each dose was 1.0 ml/kg body weight/ day, after the feeding trial, the rats were dissected and blood for serum collected for lipid profile (triglyceride, total cholesterol, LDL-c, VLDL-c and HDL-c), transaminases (ALT and AST) analysis using kits. 35 colonies from palm were studied and 22 were Gram positive. Catalase negative text allowed the selection 10 of them which were studied for their acid and bile tolerance; Data from acid and bile tolerance assays showed that seven isolates were able to maintain viability at pH 2 and to grow in a medium with 0.4% of bile salts. After in vitro assessment for their cholesterol-lowering action 3 isolates were finally selected and identified using the API 50 CHL as two L. plantarum and one L. pentosus. These strains gave rise to a significant reduction (P < 0.05) of the cholesterol level in MRS broth. In particular, L. pentosus strain lowered the cholesterol content by an average of 76.43%, compared to L. plantarum strains which lowered cholesterol content by an average of 51.25% for the isolate 2R30 and 55.22% for 2R36 repectively. The results showed no adverse effect on rats organs according to the enzyme activities and no sign of toxicity also. The probiotic strains (Lactobacillus pentosus) selected effectively demonstrated their effect on reduction of the levels of total cholesterol, LDL and VLDL in the rats blood serum. Data from acid and bile tolerance, in-vitro cholesterol reduction and in-vivo lipidemia assays showed that the dairy isolate L. pentosus can therefore be regarded as new probiotic for and could be used for functional food production.
Keywords: Probiotic Bacteria, Serum, Cholesterol, Lipoprotein, Enzyme, Bile tolerance, Acid tolerance
Cite this paper: Eurydice Flore Tiepma Ngongang , Bernard Tiencheu , Bertrand Tatsinkou Fossi , Aduni Ufuan Achidi , Dzelafen Marcel Shiynyuy , Hilaire Macaire Womeni , Zambou Ngoufack François , Isolation and Identification of Cholesterol Lowering Probiotic Bacteria from Palm Wine (Raffia mambillensis), Journal of Microbiology Research, Vol. 6 No. 5, 2016, pp. 93-102. doi: 10.5923/j.microbiology.20160605.01.
Article Outline
1. Introduction
- Palm wine is an alcoholic beverage produced from the sap of various palm tree species. The drink is particularly common in parts of West and Central Africa, South India and the Philippines. In Africa, the sap is most often taken from oil palms such as Elaeis guineensis or from Raffia, kithul or Nipa palms [1]. The dominant bacterial population of palm wine was previously described as lactic acid bacteria [2]. In general, the methods of palm wine tapping and collection of palm sap, including air and the environment as a whole, influence the microbial content of the sap [3, 4]. The wine is collected by tapping from a growing palm and this involves making a small incision in the bark about 15cm from the top of the trunk, a clean gourd is tied around the tree to collect the sap which runs into it [5]. The sap of palm tree has been shown to be a rich medium capable of supporting the growth of various types of microorganisms. Palm wine is milky white and effervescent because of the presence of live bacteria and yeast [2] resulting from natural fermentation. In a Persian version of the Old Testament, it is said that ‘Abraham owed his longevity to the consumption of sour milk’ [6]. Metchnikoff has also reported that the longevity of the Bulgarians was in part related to their consumption of large quantities of fermented milk containing lactobacilli [7]. The name probiotic comes from the Greek 'pro bios' which means 'for life' [8]. Probiotics was first used by Lilly and Stillwell to describe the 'substances secreted by one microorganism that stimulate the growth of another [9]. It is clear that, a number of definitions of the term 'probiotic' have been used over the years but the one derived by FAO/WHO, Homayouni and approved by the International Scientific Association for Probiotics and Prebiotics [10-12] best exemplifies the breadth and scope of probiotics as they are known today: 'live microorganisms which, when administered in adequate amounts, exert health benefits on the host'. Use of probiotic term for microorganisms requires criteria such as: the microorganism must be capable of being prepared in a viable manner and on a large scale, during use and under storage, the probiotics should remain viable and stable, they should be able to survive in the intestinal ecosystem and the host animal should gain beneficially from harboring the probiotic [13].The main characteristic of a probiotic microorganism is its resistance to digestion by enteric or pancreatic enzymes, gastric acid, bile, ability to prevent the adherence, establishment and replication of pathogens in the gastrointestinal tract [14]. The current conventional medical treatment for obesity is cholesterol-lowering prescription drugs along with low saturated fat diets which although lower serum cholesterol, have potential side effects, unlike probiotic microorganisms which actually can reduce serum cholesterol and have no side effects.Increased cholesterol levels have been associated with many cardiovascular diseases. Despite the available treatments for hypercholesterolemia, the prevalence of obesity in Cameroon is still increasing thus in the present study, we assessed the probiotic characteristics of lactobacilli strains isolated from palm wine under conditions that stimulate the in-vivo stresses encountered in the gastrointestinal tract such as acid, alkaline, proteolytic enzyme and bile stress. The therapeutic potential of probiotics lactobacilli and yeast strains was assessed in albino rats.
2. Materials and Methods
2.1. Sample Collection and Media Preparation
- Samples of raffia palm wine that were used in this study were collected from Foto and Foreke areas of Menoua subdivision of West Cameroon and collected from 9 different palm wine tappers. The samples were collected in sterile sampling tubes. The samples were kept at 4°C and transported in cool boxes packed with dry ice to the Life Science Laboratory of the faculty of science, University Buea, Cameroon. To ensure consistency, two tappers were selected as sources of the required samples. All media were prepared following the manufacturer’s instructions. The media used were de Man Rogosa and Sharpe (MRS) (Oxoid) agar for isolation of LAB, Aseptic techniques were observed throughout the media preparation process. A 1:10 dilution of each sample was made prior to culturing. This was done by diluting 1ml of the samplewith 9ml of physiological saline (0.85% NaCl). Further, ten-fold serial dilutions ranging from 10-1 to 10-5 were prepared. The 10-5 diluted samples were used for culture on MRS Agar and Nutrient agar.
2.2. Isolation Selection and Identification of Bacteria from Palm Wine
2.2.1. Isolation and Selection
- Bacteria were isolated from seven samples of palm wine (raffia) by pour plate method using De Man Rogosa and Sharpe (MRS) agar. For this purpose, 1ml of each sample was added to 9ml of saline solution (NaCl, 0.85%). 1 ml aliquot of the 10-4 and 10-5 dilutions was aseptically disposed on sterile plates. All plates were incubated at 30°C for 48 hours under anaerobic conditions. After the incubation of the isolates, a preliminary catalase test was carried out and another isolates were selected Based on the catalase test. From the Catalase negative test, discrete colonies which appeared on the plates with distinct morphological differences such as color, shape and size were picked and purified 2-3 times by restreaking on fresh MRS agar. These pure colonies were further characterized using Gram staining test and cell morphology examinations and 10 Gram positive isolates were selected. Catalase negative and Gram positive isolates were used from acid tolerance and bile salt tolerance test and 7 isolates were selected. Among the seven, 3 isolates were able to assimilate cholesterol in vitro. The three isolates were preserved in 15% (v/v) glycerol at -80°C until identification. Carbohydrate fermentation patterns of LAB were determined using API 50 CHL kit (Bio Merieux, France) to identify selected isolates.
2.2.2. Acid Tolerance
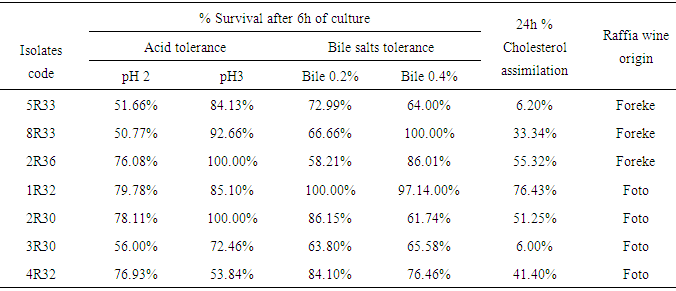
- Overnight cultures of test isolates coded 5R33, 8R33 and 2R36 originated from Foreke and 1R32, 2R30, 3R30 and 4R32 originated from Foto village were inoculated (1% v/v) in MRS broth (Oxoid) previously adjusted to pH values 2, and 3, with 1N HCl or NaOH. The cultures were incubated aerobically at 37°C for 6h. Culture turbidity was monitored at 650 nm (Pharmacia LKB, Novaspec II, England) for growth at hourly intervals. The control comprised MRS broth adjusted to pH 7. The experiment was conducted in triplicate.
2.2.3. Bile Salt Tolerance
- The method of Gilliland et al. was used to determine bile tolerance [15]. Bile-resistance was determined using the broth assay. Overnight cultures (1% v/v) of the 7 isolates (5R33, 8R33, 2R36 1R32, 2R30, 3R30 and 4R32) with 100 µl aliquot of the bacteria suspensions (107-108 CFU/ml) were inoculated in MRS broth (control cultures) and MRS broth containing 0.2, and 0.4 (w/v) bile salts oxgall (Bronadica, Hispan Lab, SA) and incubated aerobically at 37°C for 6 h. The pH of control and test cultures were adjusted to 6 with 1N HCl or NaOH. Then, viable bacteria counts were obtained after 6h incubation at 37°C 16]. The experiments were performed in duplicates. In both cases, the survival percentage of LAB was calculated by the following formula: % Survival = Final (cfu/ml) /control (cfu/ml) x 100
2.2.4. Cholesterol Assimilation from Culture Media
- Freshly prepared MRS Broth was poured into sixteen screw tubes. All the tubes were supplemented with 0.4% bile salts and 1% cholesterol but tubes 1 to 14 were inoculated in duplicate with 1 % of respective cultures (5R33, 8R33, 2R36, 1R32, 2R30, 3R30 and 4R32) while tubes 15 and 16 were free of isolate and used as control. They were incubated at 37°C for 24h. After incubation, cultures were centrifuged and unutilized cholesterol was estimated in the supernatant by measuring the optical density at 540 nm and compared to the control. Isolates having in vitro reduction of cholesterol on the media were selected.
2.2.5. Strain Identification Using the API 50CHL System
- The identification of lactic acid bacteria from the selected isolates (1R32, 2R30 and 2R36) at species level was done by biochemical characterization using the API 50CH kit (BioMerieux, France). The API 50 CH is a standardized system that associates the fermentation of 50 carbohydrates to bacteria species. It is used for the identification of Lactobacillus and related genera [17]. 10ml of pure water was dispensed into the incubation box with the strip placed in the incubation box, after the bacterial cultures had been introduced into the API 50 CHL system in API 50 CHL medium (5ml), in concentration 2McFarland. The set-up system was then incubated at appropriate temperature of 35°C for 48h, after the wells were filled with the bacterial suspensions by the line mark with the addition of mineral oil. Bacterial strains from palm wine samples with a positive test corresponds to the acidification revealed by the bromocresol purple indicator contained in the medium changing to yellow. For the esculin test, a change in colour from purple to black was interpreted as positive (+). The biochemical profile obtained for the LAB strains was analyzed using the API identification software database (API LAB PLUS), Version 5.
2.3. Invivo Evaluation of the Effect of the Probiotics Strain on the Lipid Profile
- After identification of the isolates, only the isolate (1R32) presenting best acid and bile salts tolerance and high in-vitro assimilation of cholesterol was considered for in vitro assay.
2.3.1. Animal Feeding and Experimental Design
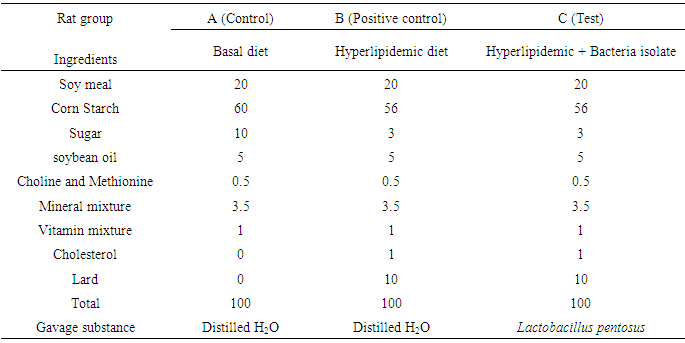
- 18 wistar albino rats (60 – 90 g) of about 21 days obtained from the animal house of the Department of Zoology, University of Buea were separated into 3 groups of 6 rats each (3 males, 3 females), the negative control group (fed with basal diet + oral gavage of deionized water) termed A, the positive control group (received hyperlipidemic diet (about 85% basal diet, 1% cholesterol, 10% lard (pig fat), W/W + oral gavage of deionized water) termed B, and a test groups (fed with hyperlipidemic diet and received bacteria isolate (1R32) termed group C. they were housed under standard conditions with a 12h light and 12h dark cycle. Temperature was maintained at 23 ± 2°C and relative humidity at approximately 50%. The rats were acclimatized for 7 days prior to use. The animals were housed in metabolic cages and given standard diet (commercial pellet formula adapted from Malathi et al. formula with slight modifications [18]) and water ad libitum throughout the study. The experiment was carried out for four weeks at oral gavages dose level 108-1010 CFU/ ml at a volume of 1.0 ml/kg body weight/day for each dose. The amount of food consumed and animal’s weight were recorded daily. The composition of the basal diet is shown on the Table 1.
|
2.3.2. Rat Blood Collection and Biochemical Assay
- After dosing (30days), the rats were allowed to fast overnight (12 hours) and on the 31st day, anaesthetized using chloroform and sacrificed. Blood was collected by cardiac puncture into eppendorf tubes and the whole blood was centrifuged for 10 minutes at 4000 rpm to obtained serum. The sera were kept at -20°C for the analysis of the lipid profile.Serum lipid profile, triglycerides (TG) were determined with the use of commercial kits. Total cholesterol (TC) was analyzed enzymatically with CHOD/PAP Method [19-21] and HDLc was also estimated with precipitation using commercial kits [22]. Serum LDLc was determined according to the Friedewald formula with use of HDLc and total cholesterol values [23]. VLDL-cholesterol (VLDL-c) was calculated by dividing triglyceride concentration by five. HDL/LDL ratio was calculated. Albumin, serum activities of alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) were measured using a commercially available kit via the methods described respectively by Tietz et al., Doumas et al. and Murray-Kaplan et al. [24-26]. An ELISA plate reader (Labsystems, MultiskanEX, Finland) was used to read the absorbance. All the kits used were manufactured by CHRONOLAB SYSTEMS in Barcelona, Spain and were used according to the manufacturer’s instructions. After the last day of administering isolates, the rats were fasted overnight for twelve hours then sacrificed. They were dissected and their blood samples collected in eppendorf tubes aseptically together with vital organs like the liver, both kidneys, spleen and heart which were aseptically handled and put in an ice bath. Analysis was done 2 weeks later to determinethe effect of the probiotic yeast isolate on the rats.
2.4. Statistical Analysis
- Data obtained were analyzed with the aid of MicrosoftR Excel 2010 software for windows. Data are presented as the mean ± standard deviation and p value < 0.05 was considered to be significant. Comparisons were made by use of the Bonferroni tests performed using Graphpad Insat 2000 software.
3. Results and Discussion
3.1. Isolation, Identification, In-vitro and In-vivo Cholesterol Lowering Effect of Isolates
3.1.1. Isolation and Selection of LAB
- Among 7 samples of palm wine, 35 colonies were isolated. Twenty two were Gram positive bacteria and among them, ten were catalase negative isolates. Acid and bile salts tolerance tests permitted the selection of 7 isolates among which three were retained for their in-vitro cholesterol assimilation on culture media and considered as presumptive LAB. Three of this genus of Lactobacillus was further identified as strains of L. pentosus (isolate1R32) and as strain of L. plantarum (isolates 2R36 and 2R30).
3.1.2. Acid Tolerance of Cultures
- The effects of acidity on the viability of isolates are presented in Table 2. 8R33 and 5R33 were the most acid sensitive of all isolates tested at pH 2. As seen from Table 2 most of the isolates tested were sensitive to acid. Less than 60% survival rate was observed at pH 2 for isolate 5R33, 8R33 and 3R30 while isolates 1R32, 2R30, 2R36 and 4R32 recorded more than 75% rate of survival at this pH. Isolates 2R30 and 2R36 showed the same behavior (high resistance with 100% survivals) under the experimental conditions at pH 3. The lower survival rate at pH 3 was recorded for isolate 4R32 (53.84%). Isolates 1R32, 2R30 and 2R36 presented high survivals rate at pH 2 as well as at pH 3. The viability of all the cultures increased for all isolates from pH 2 to pH 3 except 4R32; this lower survival rate in pH 2.0 compared to pH 3.0, was similar to results reported by other authors [27, 28]. Boke et al. [29] also reported that the viability of Lactobacillus strains was significantly reduced at pH 2.0 as compared to pH 3.0. Low pH environments can inhibit the metabolism and reduce the growth and viability of lactobacilli. Other studies also confirmed that exposure to gastric acid with pH ≤ 2 after 3 hours incubation caused a reduction in the viability count of the bacteria intensively [30, 31]. Based on the reports of Prasad et al. and Chan et al. the threshold point to state acid resistance in this research was set at pH=2 and pH=3 for six hours incubation as it stimulates bacteria residency in the stomach [30]. This is in accordance with findings of + Liong and Shah which stated that resistance at PH=3 were set as standards for acid tolerance of probiotic culture [32]. The resistant to low pH is the main criteria for probiotic strains selection [33]. Resistance to pH 3 is often used for in vitro assays to determine the resistance to stomach pH [34].
3.1.3. Growth in the Presence of Bile Salts
- Table 2 shows the effect of bile salts on the growth of the isolates. Significant variations existed among the cultures with regard to their ability to grow in MRS broth and in MRS broth supplemented with bile acids (P <0.05). 2R30, 5R33 and 3R30 were less (more sensitive) bile tolerant than all the other isolates tested at 0.4% Oxgall whereas at 0.2% bile salts, 2R36 and 3R30 were the most sensitive. In a comparison of all the other isolates, 2R36 at 0.2% Oxgall and 2R30 at 0.4% Oxgall were the most sensitive to bile acid, with significantly lower growth rates in the medium (58.21% and 61.74% respectively). The lower survival rates can be explained by the fact that after bacterial exposure to bile salts, disruption of cellular homeostasis occurred that caused the dissociation of lipid bilayer and integral protein of their cell membranes, resulting in bacterial content leakage and finally death of the cell [31]. Unconjugated bile acids, even at low concentrations, can inhibit the in-vitro growth of micro-organisms [35]. According to Gilliland et al. 0.3% is considered to be a critical concentration for screening for resistant strains [15]. Preliminary tests at 0.2% showed that 5R33 and 2R30 were able to record 72.99% and 86.17% survival after 6 hours of culture. Growth at less than 66% was observed for the rest of isolates and can be called medium tolerant isolates. The seven isolates selected were further grown in the presence of Oxgall (0.4%) while at 0.4% oxgall, 1R32 and 2R36 had high rate of survivals (high tolerant) (97.14% and 86% respectively) (Table 2) and can thus be classified as resistant isolates. Since they are tolerant to high bile salts concentration, they are beneficial to the body because according to Madani et al. high bile acid will co-precipitation in low pH environment with cholesterol rich food and facilitate the uptake of cholesterol (Cholesterol assimilation, incorporation to cell membrane or attachment to the bacterial cell surfaces, and resulting destabilization of cholesterol micelles) by Lactobacilli thereby preventing hypercholesterolemia [36]. Bile salt resistance is recommended as a suitable parameter for selecting probiotic strain [37].
3.1.4. Cholesterol Reduction from the Culture Media
- The amount of cholesterol assimilated during 24h of anaerobic growth at 37°C (Table 2) revealed wide variation among isolates as well as between trials for the same isolate. All isolates examined were able to assimilate cholesterol to some extent with the exception of 3R30 which did not grow well in the media used. The amounts of cholesterol assimilated by the cultures ranged From 6 to 76.43% with the highest assimilation observed in 1R32 isolate. No statistical significant differences were found between the cultures 5R33 and 3R30 tested with regard to theirs specific cholesterol uptake rate (P < 0.05). In the majority of cases, uptake of cholesterol was higher for 1R32, 2R30 and 2R36 Cultures indicating that they assimilated more than 50% the average of cholesterol in the media. However, this effect was not statistically significant (P > 0.05) between 2R30 and 2R36.Based on the result of this study, positive in vitro studies without using bile salts are evident [32, 38]. The highest in vitro cholesterol reduction of nearly 310-490 mg/l was reported for L. plantarum strains in the absence of bile (Gilliland et al 1985). When milk was used as the culture media in some studies, again, bile component was not added to the milk [39]. Moreover, it was shown that 1R32 isolated from palm wine can remove 76.43% of the whole cholesterol after complete growth. Cell wall binding capacity was stated to be the major mechanism involved in the latter study [40]. Ahire et al. reported that cholesterol reduction of L. helveticus cultured was attributed to the production of cholesterol oxidase [39]. Cholesterol reducing capability of 3-100 mg/l from the media was reported by other studies [41-43]. The in vitro study of cholesterol removal of Lactobacilli has been consistently used as a screening tool for selection of probiotic strains with diverse health promoting characteristics [44]. Gilliland et al. were the first to show that in vivo efficiency of Lactobacilli could be directly associated with the strains cholesterol-removal capability in the cholesterol-enriched media [38].
3.1.5. Strain Identification Using the API 50 CHL System
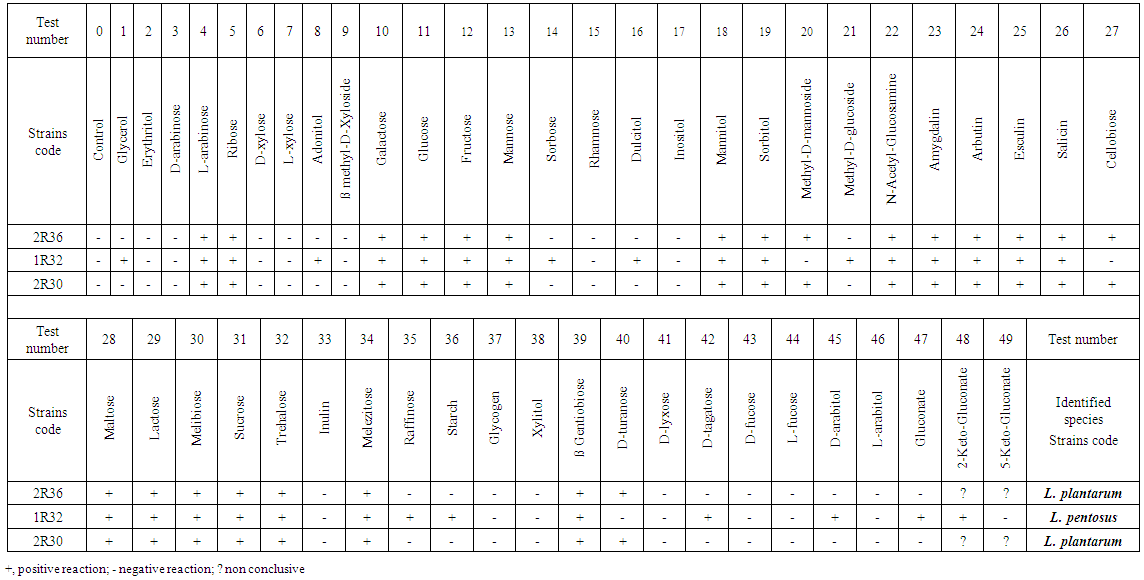
- After in-vitro test, 3 isolates of LB (1R32, 2R30, 2R36) were selected for their high in-vitro cholesterol lowering effect for identification using API 50CHL System. Utilization of carbohydrates with the API 50 CHL Kit is summarized in Table 3. Ribose, Galactose, Glucose, Fructose, Mannose, Mannitol, Sorbitol, Methyl-D-mannoside, N-Acetyl-Glucosamine, Amygdalin, Arbutin, Esculin, Salicin, Cellobiose, Maltose, Lactose, Melibiose, Sucrose, Trehalose, Melezitose, ß Gentiobiose and D-turanose were utilized by Lactobacillus sp (2R36). LB 2R30 showed higher utilization rates to those same carbohydrates.
|
 | Table 3. API test results after 48 hours of incubation |
3.2. Rats Bioassay
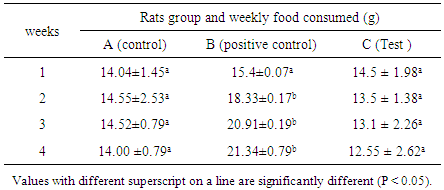
3.2.1. Feed Consumption of Rats
- Rats feed consumption is presented in Table 4. It was significantly increased in the group B but no significant effects were observed in feed consumption in the remaining treated groups. The highest and increased feed consumption in hyperlipidemic group compared to control and test groups can be due to the high fat content of the hyperlipidemic diet. Lipids are known to increase palatability of food as well as taste [46].
|
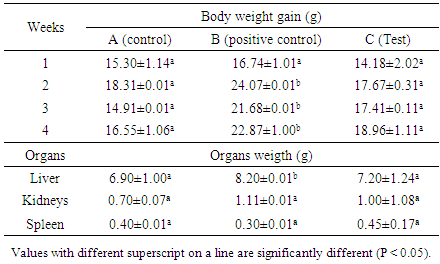
3.2.2. Weight Gain and Organs Weight
- Table 5 shows the weekly weight gain of rats during feeding period. The table revealed that the highest increases were recorded in the groups of rats fed with hyperlipidemic or hypercholesterolemic diets. These increases were not significant (P> 0.05.) between test and control groups. The test group receiving hyperlipidemic diet and the bacteria strain recorded the highest body weight. This can be due to the administration of L. pentosus that will limit weight gain probably by preventing the absorption or assimilation of cholesterol or lipids as reported by Ahire et al. [39].Rat body and organs weight gain are presented Table 5. Body weight gain was significantly high in the group B at the end of the treatment compared to the control group. No significant effects were observed in body weights in the other treated groups.
|
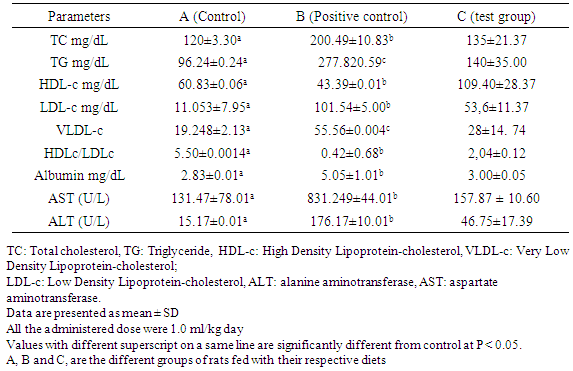
3.2.3. Serum Lipid Profile of Rat that Received L. Pentosus
- Table 6 shows the serum lipid profile of rats and two transaminase enzyme activities (ALT and AST). This table reveals that the goup of rats that received daily oral administration of bacterial culture significantly reduced cholesterol, triglyceride levels, VLDL and LDL cholesterol, while increasing HDL levels. For many years, it has been recognized that elevated serum cholesterol is a risk factor associated with atherosclerosis and coronary heart disease, the latter being a major cause of death in Western countries [48]. Numerous drugs that lower cholesterol have been used to treat hypocholesterolemic individuals. However, the undesirable side effects of these compounds have raised concerns about their therapeutic use. Ingestion of probiotic (beneficial for health) lactic acid bacteria (LAB) would possibly be a more natural method to decrease serum cholesterol in humans. This result is similar to previous studies done by Taranto et al. which demonstrated that Lactobacillus reuteri administered in low doses has a hypocholesterolemic effect both therapeutically and preventively [48].The results of this study are also similar to those carried out by Abdolamir et al. who found out that probiotics influences lipid profile parameters [49]. The increase of total cholesterol and TAG were most observed with the group thatreceived the bacteria strain. Hyperlipidemic group B which did not receive yogurts registered higher amounts of cholesterol and TAG. These results show that the declines are due to the presence of the ferment (LB bacterial).
|
4. Conclusions
- Palm wine generally refers to a group of alcoholic beverages obtained by fermentation from the saps resulting from the prolific growth of diversity of fermenting organism with probiotics characteristics. Finally, bile tolerance is considered to be an important characteristic for a probiotic that enables it to survive and then grow and exert its action in the small intestine. The L. pentosus strain used in this study was found previously to have a high level of bile tolerance and acid resistance. Therefore, as with other lactobacilli strains, this strain can be considered intrinsically resistant to human upper gastro intestinal transit. In vitro and in vivo studies should be regarded as an indicative value in the investigation of the hypocholesterolemic effect of the probiotic strain L. pentosus. The dairy isolates belonging to the species of L. plantarum and L. Pentosus strains, also grew with bile salts and low pH, they could be considered as probiotics establishing the effective cholesterol absorption property after acid and bile salts exposure and can be used to administer functional foods to hypercholesterolemic patients.
ACKNOWLEDGEMENTS
- The authors are very thankful to Glory Mba Biotechnologies Unit, Cameroon, for critical reading of the manuscript and the University of Buea, Cameroon, for supporting us by providing apparatus and reagents.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML