-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(4): 82-91
doi:10.5923/j.microbiology.20160604.03

Viability Enhancement of Schizosaccharomyces pombe Cells During Desiccation Stress
Gemma Roca-Domènech , Gema López Martínez , Victor Tirado , Anna Borrull , Óscar Candelas , Nicolas Rozès , Ricardo Cordero-Otero
Department of Biochemistry and Biotechnology, University Rovira i Virgili, Tarragona, Spain
Correspondence to: Ricardo Cordero-Otero , Department of Biochemistry and Biotechnology, University Rovira i Virgili, Tarragona, Spain.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In winemaking, the sequential inoculation of grape must with different oenological yeasts has been widely used due to the accessibility of different types of active dry yeast (ADY). Schizosaccharomyces pombe is of special interest for winemakers due to its ability to metabolize L-malic acid. Therefore, the availability of Schiz. pombe as an ADY will provide a new biotechnological tool with the same effectiveness as current commercially available oenological yeasts. In the present study, the features of metabolites were analyzed during the dehydration-rehydration process for different Schiz. pombe strains to determine whether these metabolites might play a positive role in ensuring cell viability before inoculation into the must. Our results show that the viability of cells dried in the presence of 10% trehalose and rehydrated in a solution complemented with 5 mM MgSO4 was enhanced by up to 70% for certain strains. No significant change in fermentation behavior and main volatile compounds were detected in the wines obtained with Schiz. pombe ADY in sequential- and co-inoculated with Saccharomyces cerevisiae grape must fermentation at laboratory scale.
Keywords: Schizosaccharomyces pombe, Active Dry Yeast, Cell viability, Wine
Cite this paper: Gemma Roca-Domènech , Gema López Martínez , Victor Tirado , Anna Borrull , Óscar Candelas , Nicolas Rozès , Ricardo Cordero-Otero , Viability Enhancement of Schizosaccharomyces pombe Cells During Desiccation Stress, Journal of Microbiology Research, Vol. 6 No. 4, 2016, pp. 82-91. doi: 10.5923/j.microbiology.20160604.03.
Article Outline
1. Introduction
- At present, most of the wine produced worldwide is made from musts inoculated from selected wine yeasts called active dry wine yeasts (ADWY) that are often traded in dried form [1]. The ability of Saccharomyces cerevisiae to withstand dehydration and subsequent rehydration allows for the production of ADWY inocula, ensures that fermentation will start by increasing confidence in the strain identity and genetic stability at room temperature, and reduces the costs of transportation and storage [2]. ADWY production involves dehydration of the yeast biomass to a final product with a residual moisture percentage of below 8% [3]. Many S. cerevisiae strains with an optimum oenological profile are excluded from commercial catalogues due to the sensitivity to the drying treatment. Studies have investigated the dehydration behavior exhibited by sensitive strains [4, 5]. In contrast, few studies have investigated increases in dehydration tolerance to drying and rehydration by wine yeast strains; most of these studies were performed on representatives of the genus Saccharomyces [3, 6-9]. Previously, only non-Saccharomyces yeast strains had been considered responsible for organoleptic defects in wine, but studies have reported that the sequential growth of different genera of non-Saccharomyces wine yeasts, such as Hanseniaspora, Kloeckera, Candida, Pichia, Zygosaccharomyces, Schizosaccharomyces and Torulaspora, may provide a greater aromatic complexity that is attributable to the production of secondary metabolites such as glycerol, 2-phenylethyl acetate and isoamyl acetate [10-13]. To date, a few non-Saccharomyces ADWYs, such as the Torulaspora delbrueckii 291 and Metschnikowia pulcherrima L1781 strains (Lallemand), have achieved sufficient quality to ensure good proliferation under wine-making conditions [14, 15]. Winemakers have a special interest in Schiz. pombe because of its high fermentative power, capacity to reduce the gluconic acid content of must and ability to efficiently metabolize L-malic acid into ethanol and carbon dioxide; these characteristics differentiate it from the other Saccharomyces strains [16-18]. Additionally, co-inoculation with S. cerevisiae has been reported to prevent the off flavors generated by the production of H2S, acetaldehyde, acetoin and ethyl acetate [19, 20]. In this process, fresh, immobilized Schiz. pombe cells are used for the partial or total consumption of L-malic acid before being removed to prevent off-flavor production [21]. The availability of active dry yeast of Schiz. pombe strains has provided winemakers with a new biotechnological tool with the same effectiveness as commercial ADWYs.In the present study, a protocol that transforms dry and rehydrated Schiz. pombe strains into active dry yeast was developed. Our results showed that considering the cellular physiological state before drying and initiating the rehydration process in a physiological solution improved the viability of Schiz. pombe strains isolated from grape juice by up to 70%. The consequences of drying a Schiz. pombe strain for fermentative performance was investigated. The results obtained showed that the treatment to obtain ADWY of Schiz. pombe improved cell viability without affecting fermentation efficiency and metabolic behavior.
2. Materials and Methods
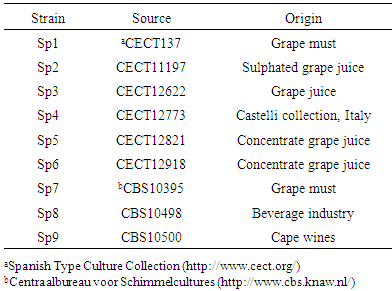
2.1. Yeast Strains and Growth Conditions
- Table 1 summarizes the Schizosaccharomyces pombe strains used in this study. The yeast strains were grown in shaker flasks at 100 rpm in EMM (Edinburgh Minimal Medium) at 32°C, inoculated with an overnight liquid culture at an initial OD600 of 0.25, and measured by microscope cell counting.
|
2.2. Desiccation-Rehydration Process
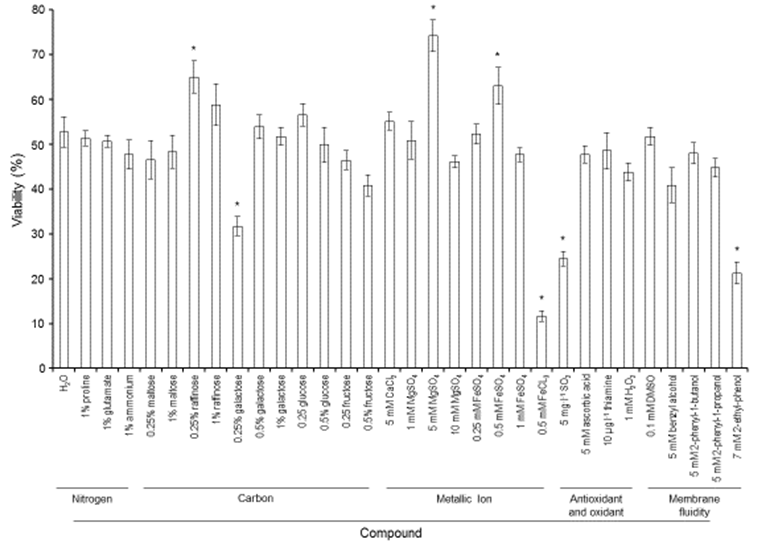
- Growth curves were determined, and 5 x 107 yeast cell suspensions, measured by microscope cell counting during the stationary phase, were desiccated in the presence of several trehalose concentrations (5%, 10%, 20% and 30%) by exposure to dry air at 28°C for ~20 h [22]. In all cases, 5 x 107 cells were rehydrated into a 1-ml final volume of water. Various rehydration temperatures (25°C, 30°C, 37°C and 40°C) and times (5 min, 15 min, 30 min and 45 min) were tested using pure water as a control. The effect of rehydration solutions on dry Schiz. pombe cells after rehydration was studied by adding each compound individually to the pure water-based condition.Cell viability was determined for the following compounds: 1% proline; 1% glutamate; 1% ammonium; 0.25 and 1% raffinose; 0.25, 0.5 and 1% galactose; 0.25 and 0.5% glucose; 0.25 and 0.5% fructose; 5, 10, 20 and 30% trehalose; 5 mM calcium; 1, 5 and 10 mM Mg; 0.25, 0.5 and 1 mM FeSO4; 0.5 mM FeCl3; 1 mM FeSO4; 5 mg·l-1 sulfur dioxide; 5 mM ascorbic acid; 10 mg l-1 thiamine; 1 mM hydrogen peroxide; 0.1 mM dimethyl sulfoxide (DMSO); 5 mM benzyl alcohol; 5 mM 2-phenyl-1-butanol; 5 mM 2-phenyl-1-propanol; and 7 mM 2-ethyl-phenol [3, 23-25].
2.3. Determining Yeast Viability
- After the rehydration process, the viable cell count was calculated by spreading cell dilutions using a Whitley Automatic Spiral Plater (AES Laboratoire, France) on YPD agar medium. The plates were incubated at 32°C for 48 h, and the CFUs (colony-forming units) were quantified using the ProtoCOL SR/HR counting system software version 1.27 supplied by Symbiosis (Cambridge, UK).
2.4. Tests for Intracellular ROS Accumulation
- Dihydroethidium (DHE) staining was performed as described in [26]. The samples were analyzed by fluorescence microscopy. To determine the frequencies of the morphological phenotypes revealed by the DHE staining, a minimum of 500 cells were evaluated from three independent experiments using a Leica fluorescence microscope (DM4000B, Germany). A digital camera (Leica DFC300FX) and Leica IM50 software were used for the image acquisition.
2.5. Determination of Biological Parameters
- Growth in microplate wells was monitored at 600 nm every 10 min after 20 s of shaking for 24 h at 32°C in a POLARstar OMEGA instrument (BMG Labtech, Germany). Microplate wells filled with 190 µl of YPD medium were inoculated with 10 µl of rehydrated cell inoculum measured by flow cytometry cell counting to obtain an OD600 of 0.6, which is above the minimal detection limit previously established by calibration. Blanks were determined from five independent non-inoculated wells for each experimental 96-wells plate. Three independent cultures of each strain were evaluated (six times each). Growth data from plate counts were enumerated as log10 values. The biological parameters, duplication times (DT) and lag phase times (λ) were estimated by fitting the growth curves into the model using MicroFit software (Institute of Food Research, Norwich, UK) [27].
2.6. Must Preparation, Fermentation and Sampling
- Sucrose was added to Tempranillo grape juice to raise the sugar content to 200 g·l-1. Before fermentations, the juice with a pH of 3.25 was complemented with diammonium phosphate (250 mg·l-1), L-malic (8 g·l-1) and 125 mg·l-1 dimethyl dicarbonate (DMDC). The juice was mixed and kept at 8°C for 24 h to give time for the DMDC to inhibit wild yeast and lactic acid bacteria. The effectiveness of this treatment was verified by plate counting. Fermentations were performed in 550 ml fermentation flasks filled with 500 ml of must. The must was initially inoculated with S. cerevisiae EC-1118 strain 1·107 cells·ml-1 -determined by using a Neubauer chamber- to ensure a complete fermentation of the sugars. The sequential- and co-inoculated must were inoculated with 1 ml of rehydrated Schiz. pombe Sp2 strain (± 1·108 cfu·ml-1) as determined by plate counting. All fermentations were performed in triplicated and conducted at 25°C without agitation. Samples were taken every day to test for L-malic acid, to determine sugar concentration and yeast population sizes determined by plate counting on media YPD and the selective media lysine obtained by replica plating. Volatile compounds were analysed when fermentations arrived to a containing less than 5 g l-1 of residual sugar. Major volatile wine compounds analyses were carried on by Lab for Flavour Analysis and Oenology (University of Zaragoza, Spain).
2.7. Statistical Analysis
- Results were statistically analyzed by one-way ANOVA and the Scheffé test using the SPSS 15.1 statistical software package. The statistical significance was set at p<0.05.
3. Results and Discussion
3.1. Cell viability during Different Growth Phases
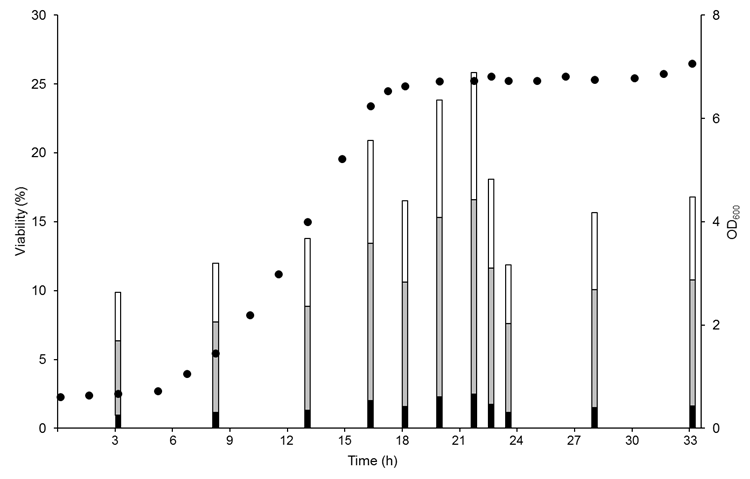
- The desiccation tolerance capacity of nine Schiz. pombe strains was assessed during growth using a colony-counting assay as described by [6] (Figure 1). After re-suspending the cells in pure water, the CFU·ml-1 mean value for survival was calculated after taking into account the cells' viability before drying. Then, the 9 strains were ranked in ascending order based on the viability rate and divided them into three arrays: Sp3, Sp5 and Sp6, <8% viability; Sp1 Sp4, and Sp7, 8-17% viability; and Sp2, Sp8 and Sp9, >17% viability (Table 1). The viability timeframe evaluated for all strains showed that the highest tolerance to dehydration occurred during the early stationary phase, between 18 h and 20 h. These results differ from previous observations for S. cerevisiae, which reached its highest desiccation tolerance capacity during the late stationary phase, before the decline phase [2].
3.2. Evaluation of Temperature and Time during Cell Rehydration
- The temperature and the time course have a direct effect on cell viability during rehydration. During this process, S. cerevisiae cells have shown a loss of up to 30% of soluble compounds due to the non-functionality of the cell membrane [3, 28]. Therefore, a more rapid functionality of the membrane may be beneficial for the viability of the rehydrated yeast cells. The temperature and time required to complete the rehydration process were correlated with desiccation tolerance. Cell viability was determined using a colony-counting assay for three Schiz. pombe strains (Sp2, Sp3 and Sp4) that demonstrated different viability rates. The viability at temperatures between 20°C and 60°C after 30 min of exposure to each temperature and at durations of exposure ranging from 5 min to 45 min at 37°C were assessed (data not shown). The incubation of cells at 55°C and 60°C resulted in a decrease of more than 80% for all strains, but no significant difference was observed between strains at temperatures between 20°C and 50°C. No statistically significant differences in the viability rate were observed for the three evaluated Schiz. pombe strains at any of the time points during incubation at 37°C. These results suggest that Schiz. pombe strains reach their best values for desiccation tolerance 10 min earlier than S. cerevisiae under similar conditions [3]. For the remainder of experiments, Schiz. pombe cells were rehydrated the by incubating them at 37°C for 5 min.
3.3. Evaluation of Cell vitality under Several Rehydration Conditions
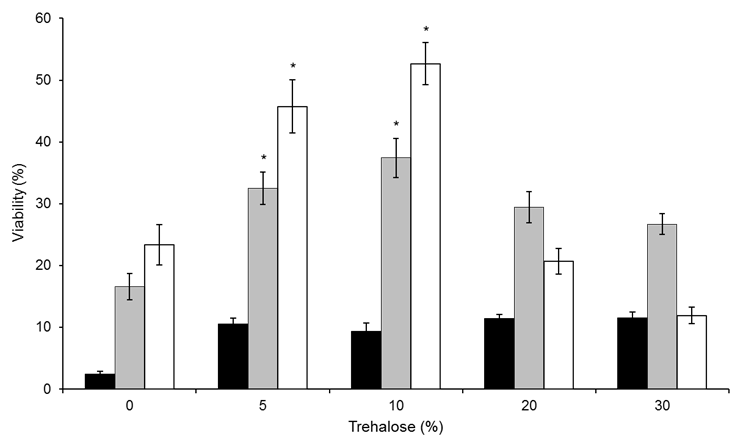
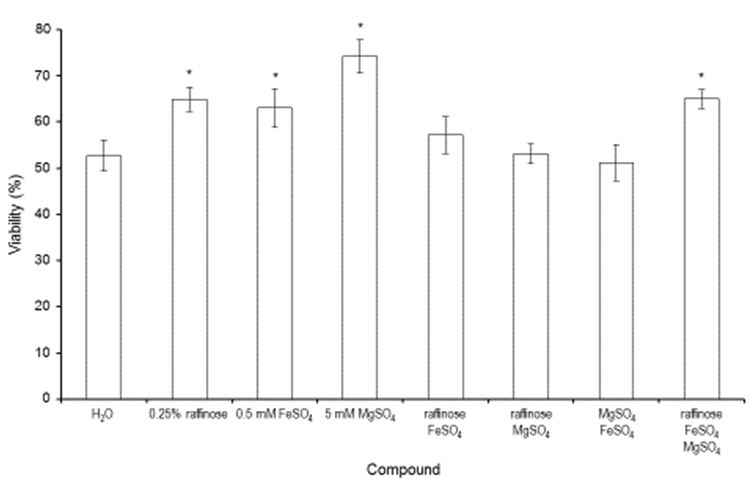
- The first attempt to enhance dehydration stress tolerance was performed by re-suspending the cells for each Schiz. pombe strain prior to the drying process in 5%, 10%, 20% and 30% trehalose; deionized water was used as the reference condition. The survival rates of the Sp2, Sp3 and Sp4 strains in water and in 20% and 30% trehalose were very low, with none of the strains exhibiting greater than 30% viability (Figure 2). However, the Sp2 and Sp4 strains showed a significant increase in cell viability of approximately 30% and 20% when re-suspended in 5% and 10% trehalose, respectively, compared with the pure water control.
3.4. MgSO4 Prevents Cellular ROS Accumulation
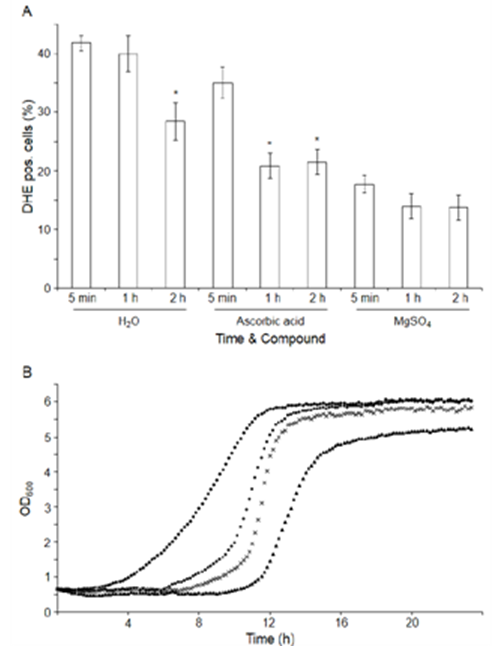
- Imposing dehydration stress by reducing intracellular ROS accumulation has been reported to enhance viability [42, 26]. Therefore, we investigated whether the higher viability rate of the cells rehydrated in the presence of MgSO4 might be due to differences in ROS accumulation [43]. After 10 min of rehydration in H2O, 5 mM ascorbic acid or 5 mM MgSO4, Sp2, Sp3 and Sp4 cells were inoculated into YPD medium. ROS-accumulating cells were evaluated over time using the dihydroethidium (DHE) assay (Figure 5A). After 1 h of incubation, the rehydrated cells in H2O exhibited approximately 40% DHE-positive fluorescence; after 2 h of incubation, 10% fewer cells were detected under intense intracellular DHE staining. A similar significant reduction in ROS accumulating-cells was observed for cells rehydrated in the presence of ascorbic acid, although this effect was observed 1 h earlier than under H2O conditions. Unexpectedly, Schiz. pombe cells that were rehydrated in the presence of MgS4O did not show any changes in fluorescence between 5 min and 2 h, with similar percentages of DHE-positive cells compared to those incubated for 1 h in ascorbic acid and 2 h in H2O. Considering the lower intracellular ROS levels of the cells rehydrated in the presence of MgSO4, our results suggest that MgSO4 allows the cells to prevent the accumulation of ROS; this process seems to occur during rehydration and very early after incubation in the YEPD medium. Therefore, the faster scavenging of ROS by cells rehydrated in MgSO4 compared to ascorbic acid might explain the 18% differences observed in cell viability (Figure 3).
3.5. Evaluation of Cell Vitality under Several Rehydration Conditions
- Next, the relatively improved rehydration conditions were correlated with a shorter lag phase (λ) once the cells were inoculated into complete medium compared to the controls. The cells rehydrated in MgSO4 and in ascorbic acid + MgSO4 showed a λ that was 208 min and 86 min longer than the controls, respectively, whereas the lag time was 220 min shorter in cells rehydrated in ascorbic acid (Figure 5B). The ascorbic acid conditions showed a 1.53-fold increase in doubling time DT compared to the controls, whereas the MgSO4 condition showed 1.51-fold decrease in DT compared to the H2O controls. However, rehydration in the presence of ascorbic acid + MgSO4 did not result in any significant differences in DT. Cells rehydrated in raffinose and FeSO4 did not exhibit any significant growth differences compared to the controls (data not shown). These results, together with the cell-accumulating ROS results, might confirm that there is no correlation between lower ROS values during stress imposition and rehydration conditions with a shorter λ phase (Figure 5A-B). Under magnesium conditions, there was an increase in the λ phase even though the accumulation of intracellular ROS stopped more quickly after stress induction. Similar variations in the λ phase were previously reported in [44], in which the reduction in the accumulation of intracellular ROS during dehydration stress was mediated by an over-expression of the encoding gene for S. cerevisiae hydrophilin.
3.6. Fermentations
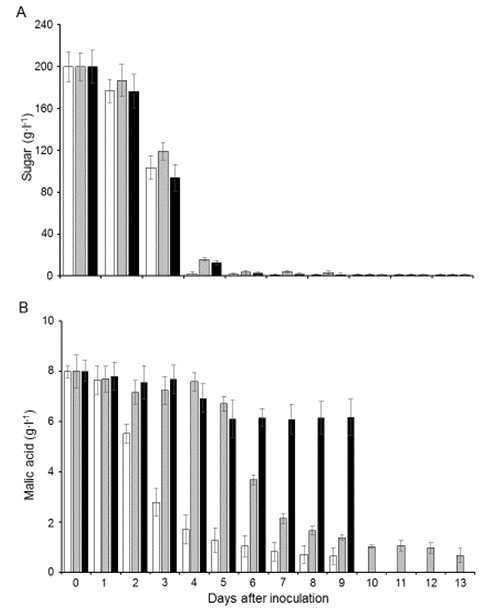
- The three treatments began soon after inoculation. The S. cerevisiae EC-1118 and the join and the sequential treatments (Schiz. pombe ADY-Sp2 and EC-1118) the wines reached values lower than 2 g·l-1 sugar in 6 days (Figure 6A). After 9 days fermentation, both double- and single-fermentations finished with an ethanol content of 12.7 ± 0.2% and 11.9 ± 0.3% v·v-1 ethanol, respectively. On day 6, prior to the addition of the rehydrated Sp2 cells to the sequential fermentations, the ethanol content was around 12% v·v-1 (data not shown). In the combined treatments, nearly 90% of the malic acid was metabolized 6 days after Schz. pombe inoculation (Figure 6B). Nevertheless, after day 9 in the EC-1118 single treatment only 12.5% of the total malic acid was metabolized. At the end of fermentation in the join and sequential treatments Sp2 cell viability was 2% and 10% respectively, while EC-1118 showed a viability of around 95% (data not shown). These data suggest that Schiz. pombe ADY is very efficient in malic acid utilization in sequential or join treatment, as previously observed using fresh inoculums [20].
3.7. Schiz. pombe ADY Metabolic Behavior was not Negatively Affected
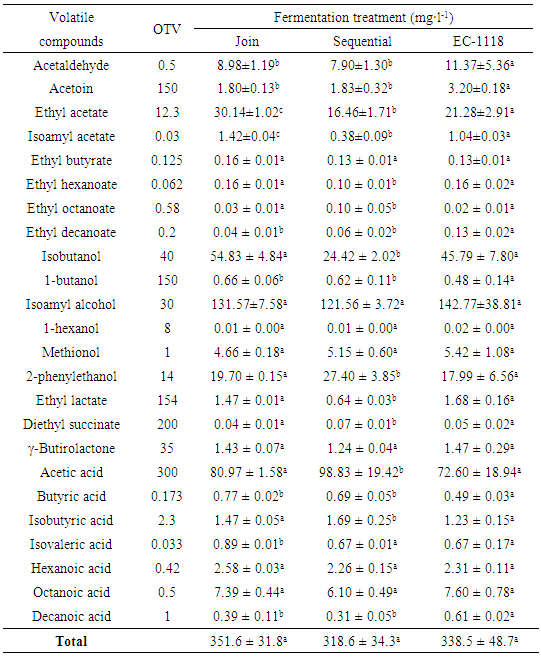
- The metabolic behavior of Sp2 ADY during fermentation was evaluated by comparing the amounts of the principal volatile compounds determined in the experimental wines after completion of alcoholic fermentation (Table 2). The three treatments did not exhibit significant differences of total volatile compounds. In fact, only ten of the 24 evaluated compounds showed some differences among treatments. Furthermore, the join treatment (EC-1118 + Sp2) shows the higher values for most compounds such as ethyl acetate, isoamyl acetate, ethyl octanoate, isobutnol, butyric acid and isovaleric acid, suggesting a major fruited or floral profile.
|
4. Conclusions
- The desiccation tolerance of wine yeasts has enabled the wine industry to work with products with more stable microbiological characteristics. Nevertheless, such products exclude yeast strains that cannot cope with the cellular stress induced during dehydration and rehydration; this topic is of great interest for the beverage industry [6]. Non-Saccharomyces yeasts, especially those with interesting oenological properties, do not often survive under desiccation stress. This characteristic is a serious handicap for winemakers who are trained to use active dry yeasts. In the present study, different Schiz. pombe strains isolated from the beverage or concentration juice industries from different regions worldwide were characterized. Isolation of the Schiz. pombe strain occurred during spontaneous fermentations without prior selection based on oenological characteristics. Previous studies have aimed to improve the tolerance of S. cerevisiae to dehydration via compound supplementation before and after stress induction [3, 7, 8, 45]. This approach led us to characterize the cell viability and vitality of nine Schiz. pombe ADWYs under a set of physiological conditions. To the best of our knowledge, this is the first systematic study to establish a protocol for developing Schiz. pombe as an ADWY. Different techniques to evaluate the influence of the compounds during the dehydration-rehydration process in the studied strains were used. The ‘fitness’ of an ADWY is related to its ability to maintain cell viability and vitality during the yeast manufacture process, including desiccation and storage [1]. In our study, yeast viability was assessed both directly - by determining the loss of cells after stress imposition and indirectly by assessing the impact of different compounds on ROS accumulation by the cells, as previously reported in [42]. The influence on cell vitality was assessed by determining the biological parameters of optimal growth conditions of the rehydrated cells. The various methods used enabled us to evaluate the effect of several rehydration conditions by uncoupling cell vitality and viability. In conclusion, the presence of both trehalose during the drying process and magnesium during the rehydration process has a synergistic effect on the enhancement of cell viability. Nevertheless, the longer lag phase promoted by the magnesium, which moves into the cell during the rehydration process, promotes sluggish cell activity. Furthermore, no defects were found between wines obtained by single S. cerevisiae and Schiz. pombe combined treatments as regard to the fermentative performance and volatile compounds influencing wine aroma. On the other hand, the malic acid was nearly completely metabolized by Schiz. pombe in combined treatments. These findings indicate that the applied protocol to obtain a Schiz. pombe as ADW yeast does not affect its oenological behavior.
ACKNOWLEDGEMENTS
- This work was supported by grant AGL2012-40018 from the Spanish Ministerio de Ciencia e Innovación. The authors thank Rovira i Virgili University for the doctoral fellowships FI-DGR 2012, AGAUR to GL and 2014PMF-PIPF-47 to GR.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML