-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(4): 69-74
doi:10.5923/j.microbiology.20160604.01

The Impact of Individual and Combined Antibiotics against Resistant Bacteria Isolated from Diabetic Foot Infection
Amal H. Hassan 1, Hassan B. Elamin 2, Suliman M. Elsanousi 3, Abdel Moneim E. Sulieman 4, Mousa M. Alreshidi 4
1Department of Microbiology, Sudan Academy of Science, Khartoum2 (Northwest Ozone) –Khartoum, Sudan
2Department of Microbial Biotechnology-Commission on Biotechnology and Genetics Engineering, National Center for Research (NCR), Khartoum, Sudan
3Department of Veterinary Medicine, University of Khartoum, Bhary-Khartoum, Sudan
4Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
Correspondence to: Abdel Moneim E. Sulieman , Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Diabetic foot infections are obviously spreading in hospital and facing unique challenge of successful treatment because of wide spread of resistant bacteria to many antibiotics. The clinical use of combination of antibiotics could be one of strategies to combat these resistant bacteria. Thus, basely depends on the microbial etiology and appropriate selective to antibiotics for combination. Using manual E-test method, Minimum inhibitory concentration (MIC) was determined susceptibility to individual antibiotics. Fractional inhibitory concentration index (FICI) was used to determine synergistic effects for combined antibiotics. Gentamicin susceptibility showed 100%, 40%, 80% and 60% of E.coli, Pseudomonas.spp, Staphylococcus aureus, and Proteus spp were sensitive, respectively. Ciprofloxacine showed 90%, 60%, 30% and 80 % resistant to E.coli, Pseudomonas spp, Staphylococcus aureus, and Proteu spp, respectively. Ceftriaxone showed 50%, 50%, 10%, and 80% resistant result against E.coli, Pseudomonas.spp, Staphylococcus aureus, and Proteus spp, respectively. Fractional inhibitory concentration index provided that 20%-40% Pseudomonas strains were synergistic to (gentamicin plus ciprofloxacin) and (gentamicin plus ceftriaxone), respectively. Since 100% of E.coli were antagonism to both combination. However, 80%-90% of Staphylococcus aureus, 70%-80% of Proteus spp showed indifferent effect to (gentamicin plus ciprofloxacin) and (gentamicin plus ceftriaxone), respectively.
Keywords: Diabetic foot infections, Antibiotics, Multidrug resistant bacteria, Antibiotic combination, Synergy
Cite this paper: Amal H. Hassan , Hassan B. Elamin , Suliman M. Elsanousi , Abdel Moneim E. Sulieman , Mousa M. Alreshidi , The Impact of Individual and Combined Antibiotics against Resistant Bacteria Isolated from Diabetic Foot Infection, Journal of Microbiology Research, Vol. 6 No. 4, 2016, pp. 69-74. doi: 10.5923/j.microbiology.20160604.01.
Article Outline
1. Introduction
- Diabetes mellitus is a metabolic syndrome characterized by hyperglycemia due to defects in insulin secretion, function, or both. Diabetic patients are more susceptible to infections; in particular foot infections which are a common and serious problem and sometimes lead to complications and amputations. Diabetic foot infections typically begin in a wound, most often due to neuropathic ulceration. Skin wounds are usually colonized with microorganisms; however, the presence of infection is defined by ≥ 2 classic findings of inflammation or purulence [1]. Selection of appropriate antibiotic for management of diabetic foot infection is crucial and largely dependent on identification of microbial pathogen and evaluation of antibiotic susceptibility [2]. Bacteria evolve to sustain survival; this entails acquisition of resistance to antibacterial, which has led to the emergence of multi-drug resistant bacteria (MDR). Infections caused by MDR bacteria carries increased the risk of morbidity and mortality worldwide, have higher financial costs and healthcare resources [3].Antimicrobial resistance is an increasing global public health problem affecting both developed and resource-limited countries; and prompted call for action worldwide [4]. Resource limited countries, including Sudan, face unique challenges in combating antibiotic resistance due to the wide availability and misuse of antibiotics without a prescription requirement and the lack of formal antimicrobial stewardship programs.In an effort to overcome MDR bacterial infections and improve clinical outcomes, optimal drug therapy is employed through combination antibiotic therapy when the identified organism is susceptible to one or more individual antibiotics. Combination of two antibacterial agents is used to enhance the drug antibacterial activity through synergistic effect. Synergy is defined as achievement of significantly greater activity by two agents combined compared to that provided by the sum of each agent alone [5]. Additionally, the use of two antibiotics prevents the emergence of resistance to either of the antibiotics if used separately as a monotherapy [6]. The purpose of this study was to define the range of resistance of bacteria isolated from diabetic foot infections and further evaluate synergistic effect of combination antibiotics to guide optimal therapy.
2. Materials and Methods
- This is a prospective cross-sectional included forty individuals presented with diabetic foot infection at Omdurman Medical Military Hospital, Omdurman, Sudan during April and May of 2015. Laboratory analysis was conducted in department of Microbiology, Tropical Medicine Research Laboratory, Khartoum, Sudan. The study focused on evaluation of the four most prevalent bacteria isolated from diabetic foot infections, which include Staphylococcus aureus, Pseudomonas spp, Proteus spp and Escherichia coli. Ten isolates of each isolated pathogen were evaluated for sensitivity testing. The study was approved by ethics committee at Omdurman Military Hospital. All participants were consented prior to enrollment in the study.
2.1. Materials
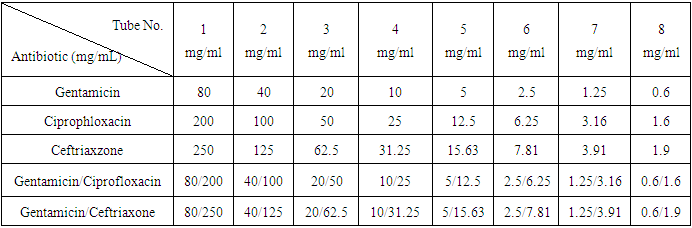
- Antibiotics used: Commercial antibiotics standard solution accompanied by a statement of its activity in mg/unit or per ml for Gentamicin (GMC company, Sudan), Ciprophloxacin (Alhekma company, Jordan), and Ceftriaxone antibiotics (Alhekma Company, Jordan), collected from the pharmaceutical seller in Khartoum. Isolates were tested against gentamicin, ciprofloxacin, ceftriaxzone, then against a combination of gentamicin with ciprofloxacin and combination of gentamicin with ceftriaxzone.
2.2. Methods
2.2.1. Production Methods of Manual E-test Method
- Two fold dilutions of the antibiotic solution in sterile normal saline were prepared for each individual antibiotic (Table 1). This was followed by impregnation via the immersion method described by Melecia et al [7], blank sterile prepared disks were soaked in the different concentration of each antibiotics for half an hour. Then the impregnated disks were transferred into sterile Petridishes. These were allowed to dry and stored in a freezer at -14°C.
|
2.2.2. The Antibiotic Susceptibility Test Procedures
- The bacterial strains isolate were collected from previously infected foot of diabetic patients. Isolated strains were homogenized in 0.85% NaCl to achieve 0.5 McFarland´s turbidity. A sterile, non-toxic swab was dipped into the inoculums suspension and pressed against the inside of the tube. Then, the surface of Muller Hinton agar in petridish was inoculated with suspension. Sterile forceps were used to deposit the antibiotic discs onto the surface of the inoculated media. Disks were made in complete contact with the agar surface, which was located in one line beginning with the highest concentration to the lowest one of the same antibiotics. The plate was then incubated at 37°C for 24 hours. After incubation, an elliptical zone of inhibition was produced and the point at which the ellipse met the defined disk concentration represented the minimum inhibitory concentration (MIC) of the antibiotics according to Clinical laboratory Standards Institute [8].Fractional inhibitory concentration index (FICI) was used to interpret the result. The FICs were calculated as follows: ∑FIC =FIC (A) +FIC (B); where FIC (A) is the MIC of drug (A) in combination / MIC of drug (A) alone. And FIC (B) is the MIC of drug B in the combination/MIC of drug (B) alone [9].
3. Results
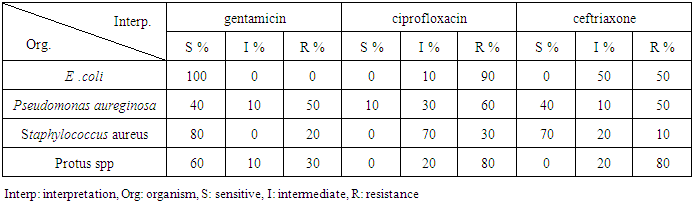
- The results showed that 100% of the E. coli strains were sensitive to gentamicin with MIC=6.6mg/ml, 80% of Staphylococcus aureus were sensitive (MIC=0.6mg/ml), only 20% were resistant (MIC=20-80mg/ml). Protus spp showed that 60% were sensitive (MIC=0.6-1.25mg/ml), 10% were intermediate (MIC=10 mg/ml) and 30% were resistant (MIC=20-80mg/ml). Pseudomonas aureginosa, showed that 50% were resistant (MIC=40-80mg/ml) and only 40% were sensitive (MIC=0.6-2.5mg/ml), 10% were intermediate (Table 2).
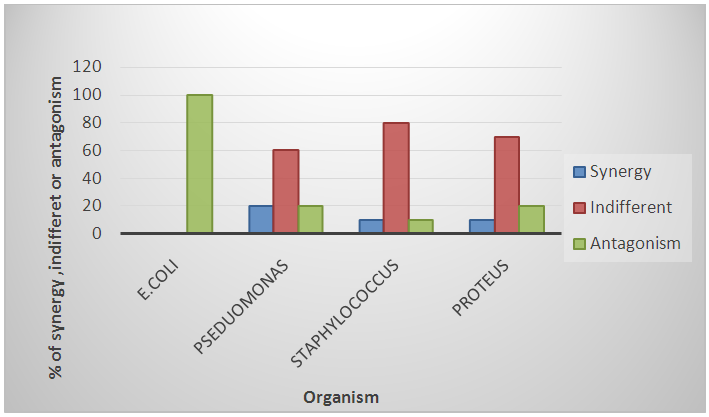 | Figure 1. Sensitivity pattern of combined Gentamicin/Ciprofloxacin on the four most common bacterial isolates from diabetic foot wounds, Omdurman Military Hospital, Sudan April-May 2015 |
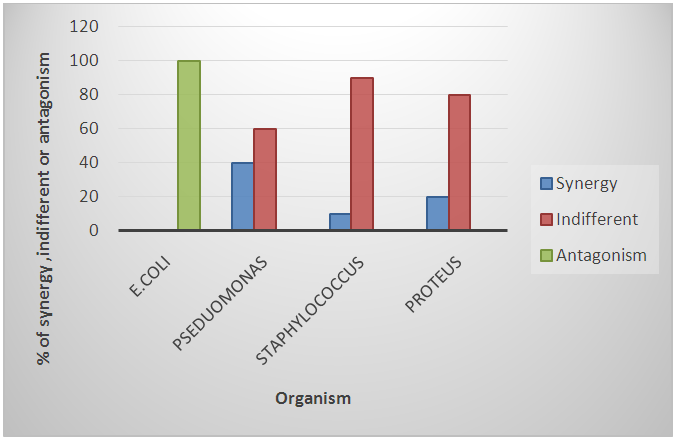 | Figure 2. Sensitivity pattern of combined Gentamicin/Ceftriaxone on the four most common bacterial isolates from diabetic foot wounds, Omdurman Military Hospital, Sudan April-May 2015 |
4. Discussion
- The aim of this study was to determine antibiotics susceptibility of four of the most commonly isolated bacteria from diabetic foot wound infection and further identify the effect of some combination antibiotics to these bacteria. Antimicrobial resistance is an increasingly serious threat to global public heath that needs urgent action. There are many factors contribute to the emergence of antimicrobial resistance and its spread in the community. These include exposure of patients to MDR bacteria while receiving medical care in healthcare facilities, inappropriate antibiotics use and lack of antimicrobial stewardship efforts, inadequate infection control measures, travel of people and goods, socioeconomic factors, antibiotic residues in the environment, bacterial gene transfer and clonal spread [10]. Our study showed that E.coli was highly sensitive to gentamicin (100%) while it was 90% resistant to ciprofloxacin. This result is similar to the finding of Kibret and Abera [11] who reported 79.6%of E.coli sensitivity to gentamicin, while recording high degree of sensitivity to ciprofloxacin. Regarding ceftriaxone, the result showed zero percent sensitivity, thus similar to study done in South Africa that tested resistant to ceftriaxone [12].Pseudomonas spp showed 40% sensitivity rate to ceftriaxone which is less than other study which gave 92.7% [13]. Gentamicin in this study inhibited 40% of Pseudomonas strains comparing with other study which recorded higher inhibition rate (87.5%) [14]. Ciprofloxacin showed high resistant rate this is similar to the study done in Saudi Arabia [15].Proteus spp showed 80% resistant to both ceftriaxone and ciprofloxacin which greatly agrees with that 83.8% sensitively re-ported by Masood et.al. [13]. Sixty percent of Proteus.spp was sensitive to gentamicin comparing with 36.8% sensitivity recorded by Saleh and Hatem [16]. Staphylococcus aurous showed 70% sensitivity to ceftriaxone. This result agrees with the study of Emmanuel and Magaji [17] who reported 71.8% sensitive. While S.aureus was resistant to ciprofloxacin, gentamicin 30%, 20%, respectively. This result is almost similar with the studies of Mounir and Bekhit [18] who reported 48.7% and 14% of S. aureus colonies resistant to ciprofloxacin and gentmicin, respectively.The resistance of these bacteria to antibiotics can be partly attributed to the ability of these bacteria to form small colony variants following the exposure to antibiotics or environmental stressors such as cold stress [19] [20]. These small colony variants were found to be more resilient and represent a survival mechanism used by many pathogenic bacteria, include S. aureus to withstand alterations in the environmental conditions [21] [22]. A recent study has found that a high number of small colony variants of S. aureus were present in diabetic foot infections [23]. This suggested that the formation of small colony variants were initiated to encounter antibiotics and changes in the environmental conditions that existed in the wound site. These changes in the phenotypes were suggested to be a consequence of adjustments in metabolic and proteomic profiles [24] [25] [26]. Combination antibiotic treatments can have significant effects on bacterial survival. Therefore, two combinations of antibiotics can be more effective in treatment of infections. The synergistic effects were observed in 20% of Pseudomonas.spp isolates to gentamicin and ciprofloxacin combination, our finding replicates the study of Yasmin et al. [27] who observed synergy in16.7% of isolates. Similarly, combination of gentamicin /ceftriaxone showed 40% synergy effect, in agreement with the study of Angehrn [28]. who recorded 67.5% synergy. Ciprofloxacin /gentamicin combination showed antagonistic effect against E.coli, this result disagrees with the study of Dickgiesser [29] who reported synergistic effect. The same authors reported indifferent effect at the combination agent to S.aureus this agrees with the present result. Proteus spp demonstrated indifferent effect to gentamicine/ciprofloxacin combination. This result disagrees with the study of Weiss [30] who reported higher susceptibility of proteus spp when subjected to gentamicin/ciprofloxacin combination. Timely access to microbiology services for identification of microbial etiology and antimicrobial sensitivity profiles is crucial to guide effective optimal antimicrobial treatment; emphasizing the importance of adoption of quality assurance measures for microbiology laboratories. Antimicrobial susceptibilities including combination drugs and their interaction would inform appropriate therapy and when adopted would minimize the emergence of antimicrobial resistance. The study focused on only on a limited number of antibiotics, and did not cover all classes. Likewise we focused on only specific range of bacterial pathogens implicated in diabetic foot infections. The scope of the study did not include clinical parameters from study participants to correlate laboratory findings.
5. Conclusions
- This study attempted to evaluate the susceptibility results of individual and synergistic effect of two combination antibiotics, gentamicin plus ciprofloxacin and gentamicin plus ceftriaxone, using manual E- test method. The individual antibiotics displayed resistance pattern to ciprofloxacin and ceftriaxone against the four isolated strains. The gentamicin antibiotic displayed synergistic effect when combined with ciprofloxacin and with ceftriaxone against Pseudomonas.aeruginosa, but it had antagonistic effect when tested against E.coli. The other two organisms, Staphylococcus aureus and Proteus spp, showed indifferent effect.Combination antibiotics strategy should be considered to target bacterial pathogens with emerging resistance pattern. Further work, coupled with molecular level studies should be done to further evaluate the synergistic and antagonistic effects among the different antibiotics combinations to maximize the efficacy of antimicrobials and prevent emergence of bacterial resistance.
ACKNOWLEDGEMENTS
- The authors express their sincere thanks to the staff and technicians of the Department of Microbiology, Sudan Academy of Science and the Department of Microbial Biotechnology, National Center for Research (NCR) for their unlimited assistance.
References
| [1] | Lipsky, B. A. et al. (2012). Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 54, e132–73 (2012). |
| [2] | Crouzet, J., Lavigne, J. P., Richard, J. L. and Sotto, A (2011). Diabetic foot infection: a critical review of recent randomized clinical trials on antibiotic therapy. Int. J. Infect. Dis. 15, e601–10 (2011). |
| [3] | Neidell, M. J. et al. (2012). Costs of healthcare-and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin. Infect. Dis. 55, 807–815 (2012). |
| [4] | DiazGranados, C. A., Cardo, D. M. and McGowan, J. E. (2008). Antimicrobial resistance: international control strategies, with a focus on limited-resource settings. International Journal of Antimicrobial Agents 32, 1–9 (2008). |
| [5] | Woods, D. E., Schaffer, M. S., Rabin, H. R., Campbell, G. D. and Sokol, P. A. (1986). Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J. Clin. Microbiol. 24, 260–4 (1986). |
| [6] | Leekha, S., Terrell, C. L. & Edson, R. S. (2011). General principles of antimicrobial therapy. Mayo Clin. Proc. 86, 156–67 (2011). |
| [7] | Windows, M. et al. (2014). No Title No Title. Uma ética para quantos? XXXIII, 81–87 (2014). |
| [8] | Clsi. (3013). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Clinical and Laboratory Standards Institute 32, (2013). |
| [9] | Meletiadis, J., Pournaras, S., Roilides, E. and Walsh, T. J. (2010). Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumi. Antimicrob. Agents Chemother. 54, 602–9 (2010). |
| [10] | Levy, S. B. (2002). Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 49, 25–30 (2002). |
| [11] | Kibret, M. & Abera, B. (2011). Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr. Health Sci. 11 Suppl 1, S40–5 (2011). |
| [12] | Lowman, W., Aithma, N., Duse, A. G. and Mer, M. (2012). Comparative MIC evaluation of a generic ceftriaxone by both microdilution on clinically relevant isolates from an academic hospital complex in South Africa. South African Medical Journal 102, 102–103 (2012). |
| [13] | Masood, S. H. and Aslam, N. (2010). In Vitro Susceptibility Test of Different Clinical Isolates against Ceftriaxone. Oman Med. J. 25, 199–202 (2010). |
| [14] | Anguzu, J. R. and Olila, D. (2007). Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. Afr. Health Sci. 7, 148–54 (2007). |
| [15] | Akhtar, N., Alqurashi, A. M. and Abu Twibah, M. (2010). In vitro ciprofloxacin resistance profiles among gram-negative bacteria isolated from clinical specimens in a teaching hospital. J. Pak. Med. Assoc. 60, 625–7 (2010). |
| [16] | Bahashwan, S. A. & Shafey, H. M. El. (2013). Antimicrobial resistance patterns of proteus isolates from clinical specimens. Eur. Sci. J. 9, 1857–788 (2013). |
| [17] | Nwankwo, E. O. and Nasiru, M. S. (2011). Antibiotic sensitivity pattern of Staphylococcus aureus from clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan Afr. Med. J. 8, 4 (2011). |
| [18] | Salem-bekhit, M. M. Phenotypic and Genotypic Characterization of Nosocomial Isolates of Staphylococcus aureus with Reference to Methicillin Resistance. 13, 1239–1246 (2014). |
| [19] | Samuelsen O, Haukland HH, Kahl BC, von Eiff C, Proctor RA, Ulvatne H, et al. (2005). Staphylococcus aureus small colony variants are resistant to the antimicrobial peptide lactoferricin B. J Antimicrob Chemother. 2005; 56(6): 1126-9. |
| [20] | Onyango LA, Dunstan RH, Gottfries J, von Eiff C, Roberts TK. (2012). Effect of Low Temperature on Growth and Ultra-Structure of Staphylococcus spp. PLoS One. 2012; 7(1): e29031. PubMed Central PMCID: PMC3265459. |
| [21] | Alreshidi MM, Dunstan HR, Roberts TK, Onyango LA. Staphylococcal phenomics: metabolomic and proteomic responses to environmental stressors. In: Méndez-Vilas A, editor. Microbial pathogens and strategies for combating them: science, technology and education. 1. Badajoz, Spain Formatex Research Center; 2013. p. 690-701. |
| [22] | Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, et al. (2004). Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(4):295-305. doi: 10.1038/nrmicro1384. PubMed PMID: 16541137. |
| [23] | Cervantes-Garcia E, Garcia-Gonzalez R, Reyes-Torres A, Resendiz-Albor AA, Salazar-Schettino PM. Staphylococcus aureus small colony variants in diabetic foot infections. Diabet Foot Ankle. 2015;6:26431. doi: 10.3402/dfa.v6.26431. PubMed PMID: 25787018; PubMed Central PMCID: PMC4365137. |
| [24] | Kriegeskorte A, Konig S, Sander G, Pirkl A, Mahabir E, Proctor RA, et al. Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics. 2011;11(12):2476-90. doi: DOI 10.1002/pmic.201000796. PubMed PMID: ISI:000292832500009. |
| [25] | Alreshidi MM, Dunstan RH, Macdonald MM, Smith ND, Gottfries J, Roberts TK. Metabolomic and proteomic responses of Staphylococcus aureus to prolonged cold stress. J Proteomics. 2015;121:44-55. doi: 10.1016/j.jprot.2015.03.010. PubMed PMID: 25782752. |
| [26] | Alreshidi MM, Dunstan RH, Gottfries J, Macdonald MM, Crompton MJ, Ang CS, et al. (2016). Changes in the Cytoplasmic Composition of Amino Acids and Proteins Observed in Staphylococcus aureus during Growth under Variable Growth Conditions Representative of the Human Wound Site. PLoS One. 2016;11(7):e0159662. doi: 10.1371/journal.pone.0159662. PubMed PMID: 27442022; PubMed Central PMCID: PMC4956324. |
| [27] | Yasmin, F., Akhtar, N. and Hameed, A. (2013). Report: In vitro synergistic Effect of Ciprofloxacin with Aminoglycosides against Multidrug resistant-Pseudomonas aeruginosa. Pak. J. Pharm. Sci. 26, 1041–4 (2013). |
| [28] | Angehrn, P. (1983). In vitro and in vivo synergy between ceftriaxone and aminoglycosides against Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. 2, 489–95 (1983). |
| [29] | Dickgiesser, N., in der Stroth, S. and Wundt, W. (1986). Synergism of ciprofloxacin with beta-lactam antibiotics, gentamicin, minocycline and pipemidic acid]. Infection 14, 82–5 (1986). |
| [30] | Weiss, D., Trautmann, M., Wagner, J., Borner, K. and Hahn, H. (1986). Ciprofloxacin: a comparative evaluation of its bactericidal activity in human serum against four enterobacterial species. Drugs Exp. Clin. Res. 12, 889–94 (1986). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML