-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(3): 55-64
doi:10.5923/j.microbiology.20160603.02

Variation in Antimicrobial Activity of Warburgia ugandensis Extracts from Different Populations across the Kenyan Rift Valley
Abuto J. O.1, Muchugi A.2, Mburu D.1, Machocho A. K.3, Karau G. M.4
1Department of Biochemistry & Biotechnology, Kenyatta University, Nairobi, Kenya
2World Agroforestry Centre (ICRAF), Nairobi, Kenya
3Department of Chemistry, Kenyatta University, Nairobi, Kenya
4Testing Services Department, Kenya Bureau of Standards, Nairobi, Kenya
Correspondence to: Abuto J. O., Department of Biochemistry & Biotechnology, Kenyatta University, Nairobi, Kenya.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Warburgia ugandensis isa highly valuedmedicinal tree within East Africa which is over-exploited for its medicinal use among many communities. This species has its habitat encroached and this has led to a notable decrease in its population size to the level that warrant some conservation efforts. Information on diversity in its antimicrobial activity is also lacking. The aim of this study was to evaluate variation in antimicrobial activity of W. ugandensis leaf and stem bark extracts from different populations across the Kenyan Rift Valley. The plant materials were collected, dried at room temperature, milled into powder and sequentially extracted with dichloromethane (DCM) and methanol (MeOH). The antimicrobial activity tests against Staphylococcus aureus, Escherichia coli and Candida albicans were carried out using disk diffusion and ninety six well microtitre plate assays. Antimicrobial activities were qualitatively and quantitatively assessed by the presence or absence of inhibition zones and minimum inhibitory concentration values. The stem bark extracts displayed the highest antimicrobial activity compared to the leaf extracts, regardless of the extracting solvents. The DCM extracts exhibited stronger antimicrobial activity compared to MeOH extracts. Staphylococcus aureus and C. albicans were sensitive to the plant extracts while E. coli was resistant. This study revealed significant differences in antimicrobial activity between extracts of W. ugandensis from different plant parts and regions (P > 0.05). The knowledge on variations in antimicrobial activity is important in developing efficient conservation and utilisation strategies for the species through identification of suitable genotypes.
Keywords: Warburgia ugandensis, Conservation, Genotypes, Variation, Antimicrobial activity, Kenyan Rift Valley
Cite this paper: Abuto J. O., Muchugi A., Mburu D., Machocho A. K., Karau G. M., Variation in Antimicrobial Activity of Warburgia ugandensis Extracts from Different Populations across the Kenyan Rift Valley, Journal of Microbiology Research, Vol. 6 No. 3, 2016, pp. 55-64. doi: 10.5923/j.microbiology.20160603.02.
Article Outline
1. Introduction
- Warburgia ugandensis Sprague is a highly valued medicinal plant belonging to the family Canellaceae that has restricted distribution in the tropical Africa (Afrotropic ecozones) [1]. The tree species is facing threat of destruction due to poor harvesting methods and over-exploitation by traditional medicinal practitioners based on its wide application in treatment and management of various diseases and use as timber. Encroachment and fragmentation of W. ugandensis habitat have led to notable decrease in its population size to the level that warrants some conservation efforts [2]. The plant is a spreading evergreen tree growing to a height of 4.5 to 40m with diameter of approximately 70cm. The stem bark can either be smooth, scaly, pale green, brown or slash pink. The leaves are simple, glossy, short, stalked, alternate, dotted with glands and lacking stipules [3]. The stem bark and leaves of W. ugandensis possess antimicrobial properties and are widely used in traditional medicine to treat bronchial infections, parasitic infections, stomachache, cough, toothache, common cold, fever, malaria, oral thrush, muscle pain, constipation, weak joints, cystitis, measles, diarrhoea and many other ailments [4, 5, 6, 7]. Many pharmacological studies have confirmed antimicrobial activities of W. ugandensis extracts [7, 8, 9]. The chemical composition of the stem bark and leaves of the tree is relatively well studied. The stem bark and leaves are rich in essential oils and several drimane sesquiterpenes such as warburganal, ugandensidial, mukaadial, salutarisolide, polygodial, isopolygodial and muzigadial amongst others [10, 11, 12, 48]. Studies have also identified alkaloid skimmianine and flavonol glycosides kaempferol, myricetin and quercetin in the species extracts [13, 14]. Thus, the medicinal efficacy of the tree species could be linked to the presence of some of these phytochemicals. Phytochemicals are natural and non-nutritive bioactive compounds produced by plants to safeguard them against external stress and pathogenic attack hence source of plant defence and survival [15]. Based on their biosynthetic origin, phytochemicals can be classified as phenolics, alkaloids, flavonoids, terpenes, saponins and tannins among others. Medicinal plants produce secondary metabolites that are responsible for their therapeutic properties but the presence of these molecules and conversely their activities are influenced by genetic and environmental factors including geographical locations, fertility of cultivar, plant part used, time of collection and season of the year [16]. Consequently, geographical variation may have some effects on the level of medicinal active compounds of plants of the same species [17]. Elsewhere, a study on the impact of geographical locations on Mentha spicata antibacterial activities revealed variation in the content of rosmarinic acid in the leaf tissues due to environmental and physiological conditions; therefore, any geographical change that commonly results in an alteration in the environment will affect the presumed medicinal activity of the plant [18]. Variation in phytochemicals could be accredited to geographical location, soil fertility and age of the plant which can ultimately impact on the quantity of secondary metabolites in plant species thereby affecting the presumed antimicrobial activities related to medicinal plants [19]. According to Lawal et al. [20], plants growing in the environment are exposed to a wide range of abiotic stress such as osmotic stress, salinity and temperature variations. These factors consequently affect their growth and metabolic processes necessary for the synthesis of a wide range of secondary metabolites that are responsible for their remarkable antimicrobial properties [16]. Despite many medicinal uses of W. ugandensis, there is limited information on variation in antimicrobial activity of its extracts from different populations across the Kenyan Rift Valley including different plant parts. Such knowledge on variation in antimicrobial activity of the plant is beneficial, not only for the search of plants showing high bioactivity, but also because such information may be of value in designing efficient conservation and utilization strategies for the species through identification of suitable genotypes. Focus may be on populations of W. ugandensis showing high bioactivity and whose parts would be more sustainably harvested than others. The aim of this study was to evaluate variations in antimicrobial activity of the leaf and stem bark extracts of W. ugandensis collected from different populations across the Kenyan Rift Valley.
2. Materials and Methods
2.1. Sample Collection
- Leaf and stem bark were collected randomly from five ecological populations of W. ugandensis across the Kenyan Rift Valley in the following forests; Kinale, Londiani, Rumuruti, Kitale and Karura (Figure 1). Leaves and stem barks were harvested from four trees per region. Debarking was done using a sharp edged machete at diameter of at the breast height (1.4m) as described by Gachie et al. [37]. The ecological and geographical details of these regions are summarized in Table 1.
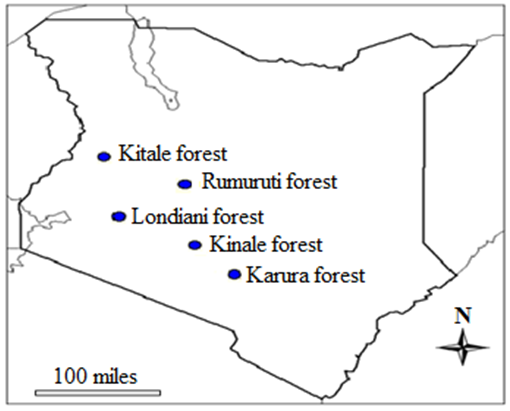 | Figure 1. Kenyan map showing regions where samples were collected |
|
2.2. Sample Preparation
- The leaves were thoroughly washed while the stem barks were cleaned with a hard brush to remove dirt and soil particles and then chopped into small pieces. They were then air dried away from direct sunlight for 2-3 weeks at room temperature until they were completely dry. The dried plant samples were ground into fine powder using an electrical mill according to standard procedures [38, 46]. The powder was kept in air tight (zip locked) polythene bags and stored at 4°C awaiting extraction and antimicrobial activity tests.
2.3. Solvent Extraction
- A 200g measure of the plant powder was sequentially extracted using dichloromethane and methanol solvents as described by Mwitari et al. [45] and Gaya et al. [46]. An 800ml aliquot of DCM was added into the conical flasks and the flasks placed on a shaker and soaked for 48 hours. The samples were filtered using Buchner funnel and Whatman filter paper (No.1) under vacuum. The filtrates were soaked in 800ml of DCM for 24 hours until they remained clear. The solution was then concentrated at reduced pressure using a rotary evaporator (Eyela-Tokyo Rikakikai Company Ltd) at 35°C and 45°C for DCM and MeOH extracts, respectively. This procedure was repeated sequentially using MeOH for the same plant samples.
2.4. Test Strains and Standard Drugs
- The antimicrobial activity tests were carried out at the Centre for Microbiology Research (CMR) at Kenya Medical Research Institute (KEMRI) in Nairobi. Pathogens used during the tests were Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922) and Candida albicans (ATCC 90028). Chloramphenical 30µg per disk (BioMerieux) and fluconazole 25µg per disk (Pfizer) were used as positive controls for the antibacterial and antifungal assays, respectively as outlined by Mwitari et al. [45]. Dimethyl sulphoxide (DMSO) (Sigma-Aldrich) was used as a negative control.
2.5. Preparation of Extracts for Antimicrobial Activity
- The extracts for bioassay analysis were prepared by transferring a 1 g of extract into pre-labeled sterile universal bottles and 1 ml of 99.9% pure DMSO added, and the mixture was consequently agitated using mechanical vortex mixer. The concentration of extracts after dissolving in DMSO was 1:1 (1 g/ml; 1 g of extract in 1 ml of DMSO). This procedure was repeated for all the extracts.
2.6. Media and Plates Preparation
- Mueller-Hinton Agar (MHA) (Oxoid, UK) was used to perform susceptibility test as outlined by Lalitha [41] and Coyle [42]. This was prepared by suspending 19g of the agar in 500ml of distilled water. The medium was boiled to dissolve in a warm water bath with continuous shaking. It was sterilized by autoclaving at 121°C for 15 minutes. The sterilized agar was allowed to cool to 50°C in a water bath. The freshly prepared and cooled agar was then poured into pre-labeled sterile petri dishes (90mm in diameter) on a level, horizontal surface to give uniform depth of 4mm corresponding to approximately 25ml of the medium per plate. The agar plates were allowed to set at room temperature. The plates were labeled by indicating the laboratory I.D numbers corresponding to the extracts.
2.7. Preparation of McFarland Standard for Bacterial Suspension Turbidity
- The 0.5 McFarland turbidity standard was prepared under sterile conditions by adding 9.95ml aliquot of 1% sulphuric acid to 0.05ml of 1.175% aqueous barium chloride [23, 40].
2.8. Preparation of Inocula Suspensions
- Test microorganisms were obtained from the stock culture maintained at CMR-KEMRI laboratory. Inocula suspensions were prepared by direct colony method [41, 42]. The microbes were grown in Mueller-Hinton Agar (MHA). 3-5 colonies of each test organism were picked with a sterile wire loop and suspended in 15 ml of sterile 0.85% saline (NaCl) in universal bottles and then mixed by mechanical vortex mixer. Thereafter, the inocula suspensions were standardized with 0.5 McFarland turbidity standards which corresponded to approximately 1.5 x 108 CFU/ml as shown by Buller et al. [40]. The turbidity of inocula suspensions were visually compared to McFarland standard by placing the tubes in front of ‘Wickerham’ card (a white card with black lines printed on it). Suspensions of similar turbidity to the 0.5 McFarland blurred the black lines to about the same extent. This process was carried out in sterile conditions.
2.9. Bioassay Screening of W. ugandensis Extracts
- A preliminary screening for antimicrobial activity of DCM and MeOH leaf and stem bark extracts of W. ugandensis was carried out using disk diffusion method according to the guidelines established by the CLSI [44]. The bacterial and fungal suspensions were inoculated onto the entire surface of MHA plates with sterile cotton-tipped swab to form an even lawn. This procedure was carried out separately for each test organism. Sterile paper disks (6mm in diameter) were impregnated with 20µl of crude extract and then placed on the surface of each MHA plate corresponding to the sample I.D numbers using sterile forceps. Chloramphenical of 30µg per disk and fluconazole of 25µg per disk were used as standard drugs for antibacterial and antifungal assays, respectively. DMSO was used as a negative control. After inoculation and application of the disks, the plates were left at room temperature for about 10 minutes to allow the extracts to diffuse from the disks to the medium. Thereafter, the plates were incubated at 37°C for 24 hours for the bacterial strains and 24-72 hours at 37°C for the fungal strain as illustrated by Maobe et al. [43]. The inhibition zones were measured to the nearest mm using a metric ruler and then the diameters were recorded as the zones of inhibition.
2.10. Minimum Inhibitory Concentration of W. ugandensis Extracts
- Prior to MIC assays, the extracts were diluted by two-fold serial dilution. Using a micropipette, 100µl of DMSO (diluent) was dispensed to all the wells across the rows of 96 well microtitre plates. 100µl of 1g/ml of the extract was transferred to the first well and mixed by using micropipette to draw up the mixture and expel it back to the well until it was homogenous. This yielded a 1:1 concentration in the first well containing the sample and DMSO. Then, 100µl of the contents of the first well was transferred to the second well in the row already containing 100µl of DMSO. Dilution and mixing was repeated for all the wells while reducing the concentration by half across the rows of microtitre plates. Then, 100µl of the contents of the last well was withdrawn and discarded, leaving all the wells with uniform volume of 100µl. This process was repeated for all the samples. Series of two-fold serial dilutions of the extracts were prepared in concentrations of 1:1 to 1:16384 for both S. aureus and C. albicans as shown by Jorgensen and Ferraro [47]. Sterile paper disks impregnated with 20µl of crude extracts in the prepared extracts dilutions were prepared. These were then placed on the surface of each MHA plate corresponding to the sample I.D numbers by use of sterile forceps. The plates were then incubated at 37°C for 24 hours for S. aureus and 24-72 hours at 37°C for C. albicans. Zones of inhibition were measured to the nearest mm using a metric ruler and recorded. Then, MIC values were determined by correlating the minimum diameter of zone of inhibition with the lowest concentration of the crude extracts at which no visible microbial growth was observed as detailed in the OIE Terrestrial Manual [39].
2.11. Statistical Analysis
- Descriptive statistics for preliminary bioassay screening of the extracts and minimum inhibitory concentrations were presented in tables and bar charts. The tests were carried out in triplicate and the mean values of the triplicate tests were recorded. All the values were expressed as mean ± standard error of mean (x ± SE). One way ANOVA was used to compare mean MICs of DCM and MeOH extracts from different plant parts and different regions against S. aureus and C. albicans. Student t-test was used to compare differences in mean MICs of the extracts against S. aureus and C. albicans. Tukey’s HSD test (post-hoc test) was used to determine significant differences between mean MICs of different plant part extracts and regions tested against S. aureus and C. albicans (P < 0.05). IBM SPSS statistics version 20 was used for all analyses.
3. Results
3.1. Bioassay Screening of W. ugandensis Extracts
- The screening results indicated that S. aureus and C. albicans were susceptible to the plant extracts while E. coli was resistant. Chloramphenical and fluconazole (positive controls) gave zones of inhibition of 20mm, respectively while DMSO (negative control) showed no activity. The stem bark extracts were the most active compared to the leaf extracts regardless of the solvents used for extraction. For instance, Kitale MeOH stem bark extracts exhibited inhibitory activity against S. aureus with mean zone of inhibition of 20 mm. This was followed by Karura MeOH stem bark extracts which showed activity with mean zone of inhibition of 18mm against S. aureus (Figures 2-5).Similarly, Kitale and Rumuruti DCM stem bark extracts exhibited inhibitory activity against S. aureus and C. albicans with mean zone of inhibitions of 19.75 mm, respectively. These were closely followed by Karura and Kinale DCM stem bark extracts with mean zones of inhibition of 18.25 and 18mm against S. aureus, respectively. The leaves of W. ugandensis showed weak to moderate activity for DCM and MeOH extracts across the five populations. For instance, Kitale and Karura DCM leaf extracts exhibited activity with mean zones of inhibition of 13.5 and 12.5mm against C. albicans and S. aureus, respectively (Figures 2-5).
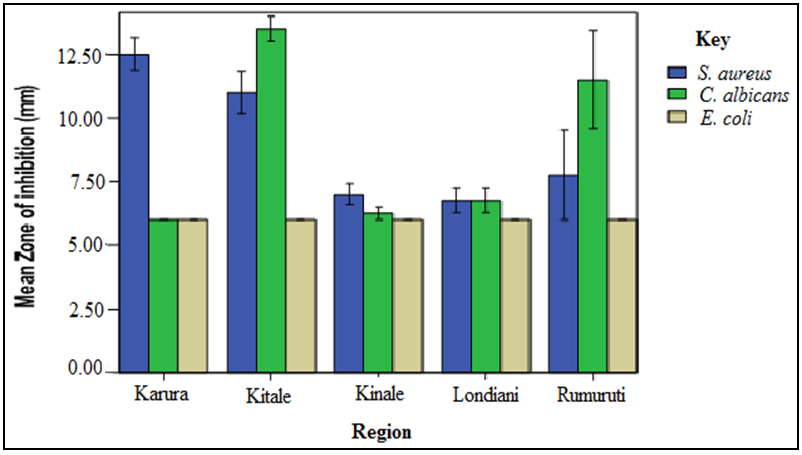 | Figure 2. Comparison of mean zone of inhibition of W. ugandensis DCM leaf extracts |
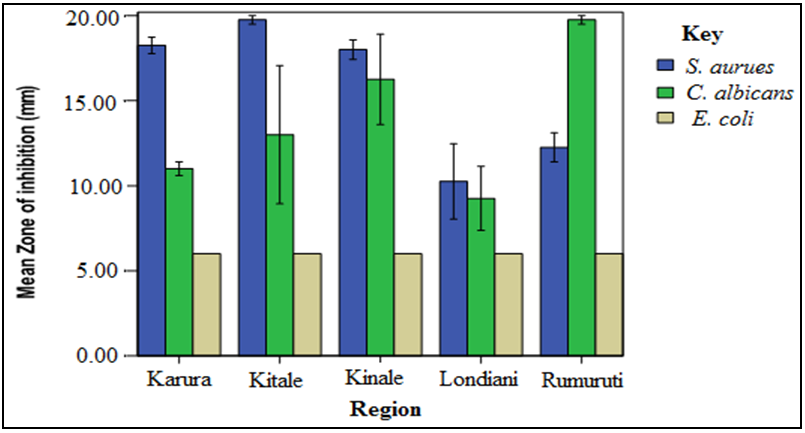 | Figure 3. Comparison of mean zone of inhibition of W. ugandensis DCM stem bark extracts |
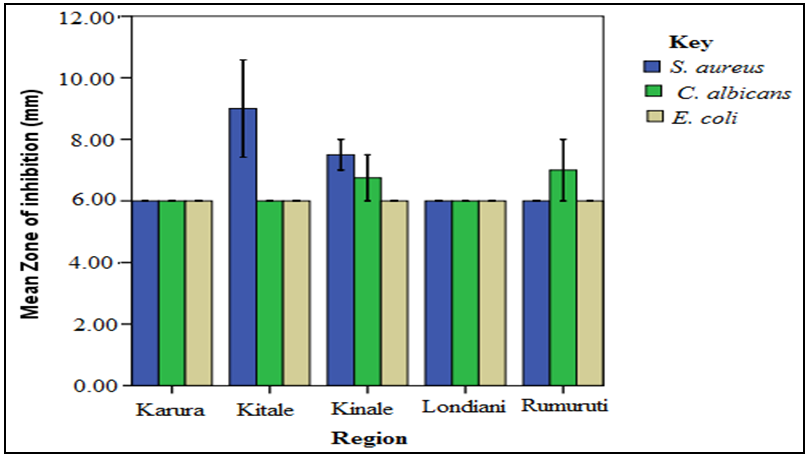 | Figure 4. Comparison of mean zone of inhibition of W. ugandensis MeOH leaf extracts |
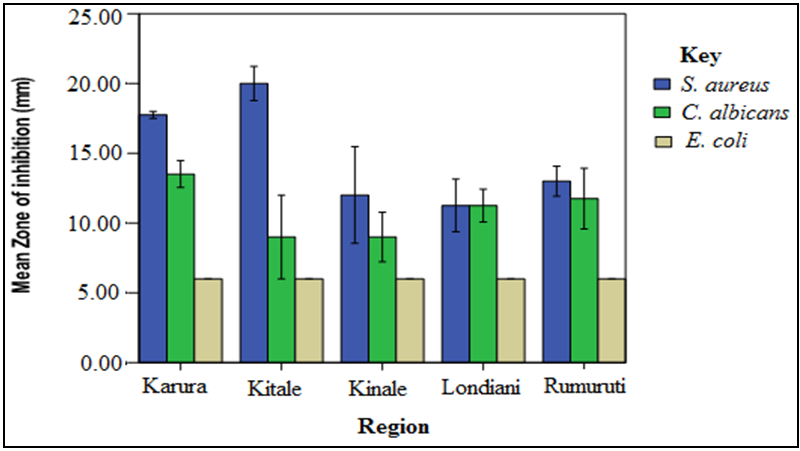 | Figure 5. Comparison of mean zone of inhibition of W. ugandensis MeOH stem bark extracts |
3.2. Minimum Inhibitory Concentration of W. ugandensis Extracts
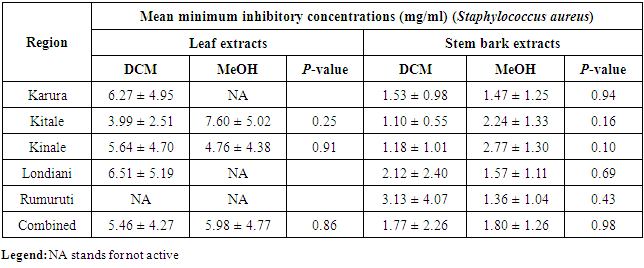
- The MIC analyses for the antibacterial effects showed that Kitale and Kinale DCM stem bark extracts were the most active against S. aureus with mean MICs of 1.10 ± 0.55 and 1.18 ± 1.01mg/ml, respectively. These were followed by Rumuruti MeOH stem bark extracts with mean MIC of 1.36 ± 1.04mg/ml against S. aureus. Karura MeOH and DCM stem bark extracts showed activity with mean MICs of 1.47 ± 1.25 and 1.53 ± 0.98mg/ml against S. aureus, respectively. Notable activity was also observed in the combined antibacterial effects of the five populations with mean MICs of 1.77 ± 2.26 and 1.80 ± 1.26 for both DCM and MeOH stem bark extracts against S. aureus, respectively (Table 2). One way ANOVA followed by Tukey’s post hoc analysis revealed a non-significant variation (P > 0.05) for both DCM and MeOH leaf and stem bark extracts for the five populations tested against S. aureus. Similarly, independent t-test comparing mean MICs of DCM and MeOH leaf and stem bark extracts for the different regions showed a non-significant difference (P > 0.05) when tested against S. aureus (Table 3).
|
|
4. Discussion
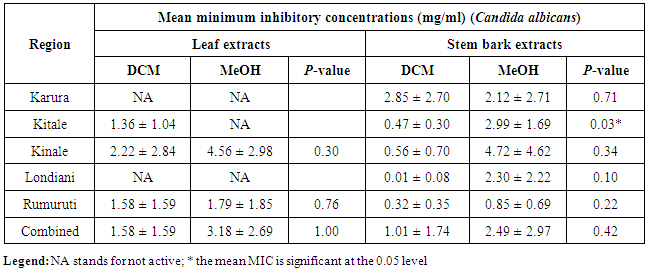
- The preliminary bioassay results showed that S. aureus and C. albicans were the most susceptible microorganisms while E. coli was resistant. This was confirmed by mean MICs of 3.17 ± 0.27 and 1.76 ± 0.21mg/ml against S. aureus and C. albicans, respectively regardless of the sites, samples, plant parts, solvent types and the extraction technique. This observation is in agreement with the work of [24] who noted that S. aureus and C. albicans were the most susceptible microbes to the crude extracts of W. ugandensis from Ethiopia. Considering the observed difference in antibacterial (3.17 ± 0.27 mg/ml) and antifungal (1.76 ± 0.21mg/ml) effects, it is worth noting that the antimicrobial activity was more pronounced against C. albicans than S. aureus. The variation in sensitivities could be attributed to the reinforced defence mechanisms acquired by both types of microorganism [25]. The differences observed in the susceptibility of S. aureus, C. albicans and E. coli to the plant extracts could be linked to the variation in diffusibility of the bioactive compounds through the culture medium [26]. The diversity in the antimicrobial activity of the extracts in this study could be explained by the fact that generally, plant extracts are more effective against Gram-positive (S. aureus) than Gram-negative (E. coli) bacteria [25]. This is likely due to the differences in morphological constitution between these microorganisms [27]. The observed morphologic variation influences the reaction of microbes to antimicrobial agents [28]. Gram-negative bacteria contain a very restrictive outer membrane with structural lipopolysaccharide components. This makes the cell wall impermeable to antimicrobial agents. On the other hand, Gram-positive bacteria are more susceptible having only an outer peptidoglycan layer which is not an effective permeability barrier [28]. Previous studies have outlined a number of possible reasons for the differences in antimicrobial activities of plant extracts. Firstly, similar plants growing in different geographical regions are known to be very different in their phytochemical profiles. Secondly, active compounds of plant species may vary significantly in their chemistry and their taxonomy in some cases is obscure. Apart from the interspecific variation in chemistry, the degree of chemical variation in the active components within a species especially from different geographical regions is still unclear. Lastly, the difference in extraction procedures could also explain the differences in bioassay results [24, 49]. The stem bark extracts were more active against the test microorganisms than the leaf extracts. This was observed in Kitale and Rumuruti DCM stem bark extracts which exhibited the highest antimicrobial activity with mean zone of inhibition of 19.75mm against S. aureus and C. albicans, respectively. These results resonates with the MIC findings for the antibacterial and antifungal effects which revealed that DCM stem bark extracts were more potent than the corresponding leaf extracts. The high bioactivity displayed by stem barks compared to the leaves could be linked to the different secondary metabolites found in these organs (stem barks) [29]. The variation in mean MICs of different plant parts (leaves and stem barks) and samples of W. ugandensis against S. aureus and C. albicans could be attributed to the difference in the exposure level of the plant extracts to the test microbes; the paper disk used in bioassays normally retains the active ingredient of the plant extracts and restrict their diffusion into the culture medium [7]. The bioactive compounds are generally accumulated as secondary metabolites in all plant cells but their concentration could vary in different plant parts [30]. In this regard, the stem bark is one of the highest accumulatory plant parts and its compounds are normally preferred for medicinal use [31]. The possible explanation for high bioactivity of the stem barks of W. ugandensis lies in the number (levels) of compounds expressed in the stem barks. It has been reported that the stem bark extracts of W. ugandensis contain relatively higher amounts of bioactive compounds particularly sesquiterpenoids that could be responsible for the antimicrobial activity and inhibition of the growth of microbes [29]. Moreover, plant components with terpenoids are known to possess antimicrobial properties [32]. The weak antimicrobial property of W. ugandensis leaf extracts could also be attributed to the lower amounts of phytochemicals expressed in the in leaves compared to the stem barks [29]. The pattern of antimicrobial inhibition of the various extracts of W. ugandensis varied with the solvent used for extraction and the part of the plant used as well as the regions where the samples were collected. It was observed that the DCM solvent extracts exhibited the highest antimicrobial activity than the MeOH solvent extracts regardless of the plant part analysed. Compared to previous studies on other plant species, Saggoo et al. [33] on their work comparing antibacterial activity of three morphotypes of Eclipta alba showed that different solvents have the capacity to extract different compounds depending on their solubility or polarity in the solvents. Therefore, the DCM solvent in this study dissolved many bioactive compounds from W. ugandensis and is therefore a very useful extractant for antimicrobial studies where terpenoids and other compounds are required to be extracted [34]. Thus, the active compounds responsible for the antimicrobial activity are more soluble in the DCM solvent extracts than the MeOH solvent extracts. Combined antimicrobial effects for the five populations of W. ugandensis across the Kenyan Rift Valley showed non-significant difference (P > 0.05) for both DCM and MeOH leaf and stem barks extracts tested against S. aureus and C. albicans. Similarly, independent t-test comparing mean MICs of DCM and MeOH leaf and stem bark extracts for the different regions showed non-significant difference (P > 0.05) when tested against S. aureus; except for Kitale population which showed significant variation (P < 0.05) when tested against C. albicans. The difference in susceptibility of the S. aureus and C. albicans could be linked to their intrinsic properties that are related to the permeability of their cell surface to the plant extracts. The observation that the extracts of W. ugandensis from Kitale region displayed variation in antimicrobial activities is in agreement with the results of an earlier study which reported diversity in biological properties of different plant species from South Africa [35]. The authors attributed the differences in the antimicrobial effect of plants to a number of intrinsic (adaptive metabolism, genetic, epigenetic) and extrinsic (environmental, eco-geographical) factors [35]. Genetic variation among different populations of W. ugandensis could lead to diversity in the medicinal properties of the plant species across the Kenyan Rift Valley [36]. Previous molecular genetics studies revealed interspecific variation (P > 0.0001) within and among populations of W. ugandensis from western (Kitale) and eastern (Karura) arms of the Rift Valley to an extent of suggesting speciation due to allopatry [2]. Genetic distinction was also observed within the Rift Valley specifically in Lakipia. These differences could be due to the geographical and genetic isolation among the populations of W. Ugandensis [2]. Similarly, the observed variation in antimicrobial activity could be related to the plant’s ecological range or environmental conditions of the forests from which the samples were collected. Therefore, it is possible to link the observed differences (P > 0.05) in antimicrobial activity of W. ugandensis populations to genetic diversity.
5. Conclusions
- The results of our study confirmed the antimicrobial activity of DCM and MeOH leaf and stem bark extracts of W. ugandensis against Staphylococcus aureus and Candida albicans. Escherichia coli was found to be resistant to the plant extracts. The stem bark extracts displayed the highest antimicrobial activity against the test microoganisms compared to the leaf extracts which showed moderate to weak antimicrobial activity. There were variations in the antimicrobial activity of W. ugandensis extracts from the five populations across the Kenyan Rift Valley with DCM stem bark extracts from Kitale, Kinale, Londiani and Rumuruti exhibiting the highest activity against the test microbes. The pattern of inhibition of the extracts against the test microoganisms varied with the solvents used for extraction and the plant part analyzed as well as the sampling sites. The DCM extracts were more active than the MeOH extracts regardless of the plant parts analyzed. Due to the high bioactivity of W. ugandensis extracts, this study advocates for sustainable utilization and conservation of all genotypes of the tree species in the genus Warburgia in different provenances of Kenya.
ACKNOWLEDGEMENTS
- This work formed part of John Abuto’s Master of Science thesis at Kenyatta University, Nairobi, Kenya. The authors are very grateful to the financial support from Warburgia ugandensis Phytochemical Research Project-IFS Research Grant number (F/3829-2). This work was also financially supported by the National Commission for Science, Technology and Innovation (NACOSTI), Kenya, through a grant number NCST/ST&I/RCD/4th Call M.Sc./157, 2012/2013.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML