-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(3): 47-54
doi:10.5923/j.microbiology.20160603.01

Extracellular Highly Thermostable α-Amylase from a Strain of Lactobacillus fermentum: Production and Partial Characterization
Frédéric Tavea1, Bertrand Tatsinkou Fossi2, Nchanji Gordon Takop2, Robert Ndjouenkeu3
1Department of Biochemistry, University of Douala, Cameroon
2Department of Microbiology and Parasitology, University of Buea, Buea, Cameroon
3Department of Food Science and Nutrition, ENSAI, University of Ngaoundéré, Ngaoundéré, Cameroon
Correspondence to: Bertrand Tatsinkou Fossi, Department of Microbiology and Parasitology, University of Buea, Buea, Cameroon.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Lactic acid bacteria are potential source of enzymes that can be used in food biotechnology because they are generally regarded as safe. A highly thermostable α-amylase producing lactic acid bacterium, Lactobacillus fermentum 04BBA19 isolated from a soil of the western region of Cameroon was characterized for its starch degrading activity and biochemical properties. The bacterium exhibited maximal production of the enzyme at temperature 45°C, and within pH range of 4.0 to 5.0. The main environmental conditions affecting enzyme productivity were the nature of substrate, the nitrogen source, the minerals content and the presence of surfactants. The enzyme was identified as an α-amylase fragment exhibiting maximum activity and stability in temperature and pH ranges of 60-70°C and 4.0-7.0 respectively, with a very high thermostability revealed by the retention of 100% of original activity after pre-incubation for 30 min at 80°C. The stability improved considerably with addition of 0.1% (w/v) CaCl2.2H2O; the half live of the enzyme under the above conditions was 6 h at 80°C.
Keywords: Lactic acid bacteria, Starch hydrolysis, Thermostable α-amylase, Fermentation
Cite this paper: Frédéric Tavea, Bertrand Tatsinkou Fossi, Nchanji Gordon Takop, Robert Ndjouenkeu, Extracellular Highly Thermostable α-Amylase from a Strain of Lactobacillus fermentum: Production and Partial Characterization, Journal of Microbiology Research, Vol. 6 No. 3, 2016, pp. 47-54. doi: 10.5923/j.microbiology.20160603.01.
Article Outline
1. Introduction
- Thermostability is one of the main features of many enzymes sold for bulk industrial usage. Thermostable α-amylases are of interest because of their potential industrial applications. They have extensive commercial applications in starch liquefaction, brewing, sizing in textile industries, paper and detergent manufacturing processes. [1-3]Several thermostable α-amylases have been purified from Bacillus sp. and the factors influencing their thermostability have been investigated, but the thermostability of amylases from lactic acid bacteria has attracted very few scientific attentions. Lactobacillus amylovorus, Lactobacillus plantarum, Lactobacillus manihotivorans, and Lactobacillus fermentum are some of the lactic acid bacteria exhibiting amylolytic activity which have been studied [4-9]. However, most of α-amylases from these bacteria presented weak thermostability compared to those of genus Bacillus.Owing to the important acidification of fermentation medium by most lactic acid bacteria, the production of thermostable amylase by a lactic acid bacterium under submerged or solid-state fermentation can help to reduce the risk of contamination caused by undesirable micro-organisms during the fermentation. [10, 11]. Another advantage is the non-pathogen character of the genus Lactobacillus that allows their utilization in food fermentation processes. The present study deals with the production and characterization of thermostable α-amylase from a lactic acid bacterium, Lactobacillus fermentum 04BBA19, isolated from starchy soil of the western region of Cameroon.
2. Materials and Methods
2.1. Origin and Nature of the Microorganism
- The starch degrading amylolytic lactic acid bacterial strains were isolated from the soil of a flour market in Bafoussam, a city of the western region of Cameroon. [12]. One of the isolates designated 04BBA19 was selected and identified using API 50 CH test kit (bioMerieux France) as Lactobacillus fermentum and used for this study.
2.2. Growth and Enzyme Production
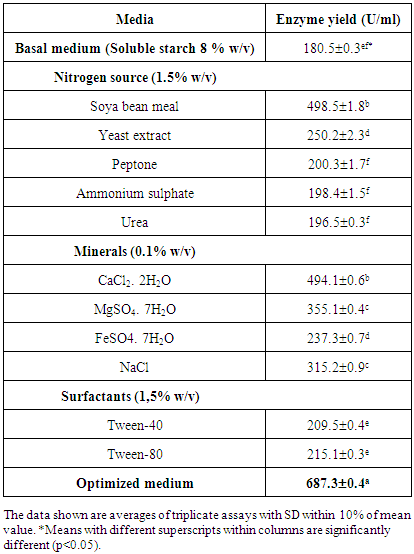
- The micro-organism was propagated at 40°C for 48 hours in 50 ml of a basal medium containing 1% (w/v) of soluble starch, 0.5% (w/v) of yeast extract placed in 100 ml Erlenmeyer flask with shaking at 150 oscillations per minute in an alternative shaker (Kotterman, Germany). The initial pH of the basal medium was adjusted to 6.5 using 0.1 M HCl or 0.1 M NaOH. Cell growth was evaluated by reading the absorbance of culture medium at 600 nm, followed by enumeration on plate counting agar. In order to evaluate the capacity of micro-organism to acidify the culture medium, the pH of the fermented broth was measured using an electronic pH meter (Mettler Seven S20, Japan). After removal of cells by centrifugation (8000xg, 30 min, 4°C) (Heraeus, Germany), the supernatant was considered as the crude enzyme solution.The amylase production was optimized by studying the effect of cultural and environmental variables (carbohydrate and nitrogen sources, metals, surfactants) individually and simultaneously. The carbohydrate sources tested were: glucose, fructose, maltose, amylose, amylopectine, cassava raw starch and soluble starch at concentration of 1% (w/v)), nitrogen sources (soya bean meal, yeast extract, peptone, ammonium sulphate and urea at concentration of 1.5% (w/v)), metal salts (CaCl2.2H2O, MgSO4.7H2O, FeSO4.7H2O, NaCl at concentration of 0.1% (w/v)) and surfactant (Tween 80 and Tween 40 at concentration of 1.5% (v/v)). All media were autoclaved at 121 °C for 20 min, while for the medium containing raw cassava; starch powder was sterilized at 120°C for 2 h in a hot oven and used as raw cassava starch for enzyme production.The effect of carbon sources was studied by replacing soluble starch with different carbohydrates.
2.3. Enzyme Purification and Assay
- The culture supernatant was supplemented with solid ammonium sulphate to 65% (w/v) final concentration, with mechanical stirring at 4°C. The suspension was retained for 1 h at 4°C, and centrifuged at 8000 g for 30 min at the same temperature. The resultant supernatant was brought to 70% w/v ammonium sulphate saturation at 4°C. 50-70% (w/v) ammonium sulphate precipitate was recovered, dissolved in 0.1 M phosphate buffer and dialysed with Spectra/PorR, VWR 2003 dialysis membrane overnight against the same buffer at 4°C.Affinity chromatography was carried out by the modified technique described by Zang et al. (1994) [13]. The modification was using of insoluble cassava starch instead of insoluble potato starch as support for the affinity column chromatograph. Before packing on the column (1,5x15cm), the cassava starch (25%) was swollen in 30 % (NH4)2SO4 at 70°C. The concentrated supernatant was applied directly to column at 4°C and washed with 0.5M NaCl to remove impurities. The amylase was eluted with 0.05 M Na2CO3 at elution rate of 3 ml x 20.min-1 at room temperature (25°C). The activity of a-amylase was assayed using modified method of Giraud et al. (1993) [14]. In a typical run, 5mL of 1% (w/v) soluble starch solution and 2mL of 0.1 mol/ L phosphate buffer (pH 6.0) were mixed and maintained at 60°C for 10 min, then 0.5mL of appropriately diluted enzyme solution was added. After 30 min the enzyme reaction was stopped by rapidly adding 1mL of 1 mol/L HCl into the reaction mixture. For the determination of residual starch, 1mL of the reaction mixture was added to 2.4mL of an iodine solution containing 3% (w/v) KI, 0.3% (w/v) I2 diluted to 4% (v/v) and its optical density was read at 620nm using a Secomam Prim Visible Light Spectrophotometer, 230 VAC. One unit of α-amylase activity (U) was defined as the amount of enzyme able to hydrolyze 1 g of soluble starch in 60 min under the experimental conditions. All the values presented are means of three replicates.An aliquot of 0.5 ml of purified enzyme preparation was subjected to react with a specific substrate (2ml containing 1g/l of blocked p- nitrophenyle maltoheptaoside). This substrate known to be hydrolysed only by α-amylase [15], a positive reaction, was characterized by the appearance of yellow compound with maximum absorption at 530 nm. In order to determine the specific activity and purification fold, protein was estimated using the method of Bradford (1976) [16] with pure casein as the standard.
2.4. Measurement of Active Component and Molecular Weight of Amylase
- To determine the homogeneity and molecular weight, the enzyme preparation and known molecular markers (Phosphorylase b: 94000 Da, Catalase: 60000 Da, Pepsine: 34700 Da, Casein: 25000 Da, Trypsin: 24000 Da) were subjected to PAGE-SDS electrophoresis using homogenized 10% (w/v) acrylamide gel. After electrophoresis, the gel was stained for 4 h with 0.25% (w/v) coomasie blue R250 dye in methanol acetic acid-water solution (50/5/45, by volume), and destained in methanol acetic-water solution (80/10/10, by volume) without dye. The SDS was then removed by washing the gel successively with distilled water and with 50 mM phosphate buffer (pH 6.0). After washing, the gel was incubated at room temperature in 50 mM phosphate buffer solution pH 6.0 for 24 h, followed by a second incubation at room temperature in 0.5% (w/v) soluble starch solution for 24 h. The amylase activity was revealed by staining in 4% (v/v) diluted iodine solution (I2, 1g/l; KI, 30 g/l).
2.5. Effect of Temperature and pH on Activity and Stability
- The optimal temperature for amylase activity was determined by assaying activity between 30 and 100°C for 30 min in 50 mM phosphate buffer. Measurement of optimum pH for amylase activity was carried out under the assay conditions for pH range of 3.0-10.0, using 50mM of three buffer solutions: Tris-HCl (pH 3.0), Na2HPO4-Citrate (pH 4.0 – 6.0), and Glycine- NaOH (pH 7.0-10.0).The temperature stability was determined by incubating the purified enzyme solution in water bath for temperature range of 30-100°C for 30, 60, 90, 120, 180 min and then cooled with tap water. The remaining α-amylase activity was measured as described above. The first order inactivation rate constants,
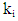 were calculated from the equation:
were calculated from the equation: 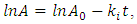 where
where 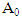 is the initial value of amylase activity and A the value of activity after a time t (min).For the determination of pH stability, the enzyme was incubated in a water bath at 60°C at varying pH for 30 min. The residual activity was detected under the same conditions and expressed as the percentage of the activity of untreated control taken as 100%.
is the initial value of amylase activity and A the value of activity after a time t (min).For the determination of pH stability, the enzyme was incubated in a water bath at 60°C at varying pH for 30 min. The residual activity was detected under the same conditions and expressed as the percentage of the activity of untreated control taken as 100%.2.6. Effect of Metal Salts and Chelating Agent
- The effect of metal salts and EDTA on amylase activity was determined by adding 0.05 to 0.1% (w/v) of metal salts (CaCl2.2H2O, MgSO4.7H2O, FeSO4.7H2O, NaCl, FeCl3, CuSO4.5H2O) and EDTA to the standard assay. The effect of metal salt and chelating agent on amylase activity was evaluated by pre-incubating the enzyme in the presence of the above effectors for 30 min at 70°C. The remaining activity was determined as described above.
2.7. Examination of the Effect of Enzyme on Raw Starch Granules
- In order to visualize the effect of enzyme on raw starch granules, 1ml of raw cassava starch suspension (0.5% w/v) was stained with iodine solution and examined by light microscopy (Olympus microscope BH-2). After treatment with 0.1 ml of purified amylase solution from Lb. fermentum 04BBA19, the examination of treated starch was carried out under the same conditions by microscopy.
3. Results and Discussion
- Enzyme productionIn the presence of starch as carbon source at 40°C, Lb. fermentum 04BBA19 strain grew and released amylolytic activity in the culture medium. Growth and amylase synthesis curves show the same profile (Figure 1). The level of amylase production increased during the exponential phase of growth. The amylase production pattern in Lb. fermemtum 04BBA19 indicates that the induction of amylase took place during the lag phase in the presence of starch. Cell growth and amylase production reached maxima values at the same time (40 h. of fermentation). The values of those maxima were 1.1x108 cfu/ml and 107.3±0.5 U/ml for cell growth and amylase activity respectively. Such coincidence shows that amylase production by Lb. fermentum 04BBA19 was tightly linked to cell growth. This phenomenon is generally attributed to the nutritional activity of the bacterium, which needs glucose for its growth. Since the extracellular medium contains starch which cannot cross the cell membrane for bacterium nutrition, cells secrete amylase for breaking down starch molecules and releasing oligosaccharides such as glucose which can then enter into the cell for the bacterium nutrition.This relationship between cell growth and amylase production was confirmed by soluble protein synthesis during fermentation (Fig. 1). Soluble protein production increased during fermentation, the highest values (0.72±0.04 mg/ml) occurred at the same time with maximum of growth and amylase activity.
|
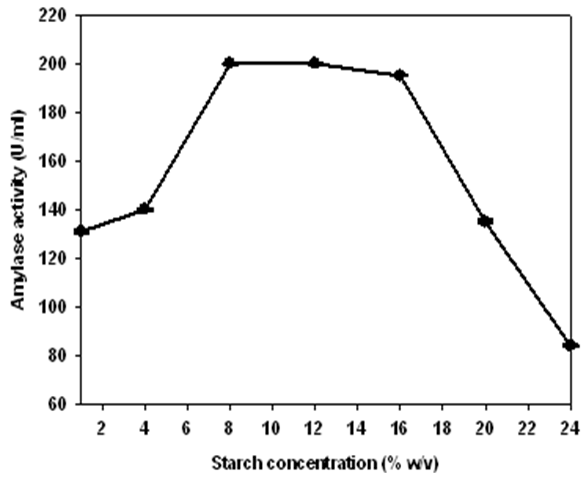 | Figure 3. The effect of starch concentration on enzyme production by Lb. fermentum 04BBA19. The data shown are averages of triplicate assays with SD within 10% of mean value |
|
|
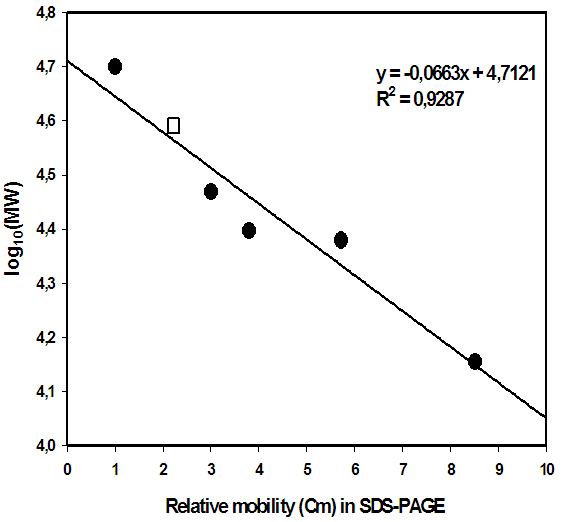 | Figure 5. Determination of relative molecular weight of α-amylase from Lb fermentum 04BBA19 in SDS-PAGE electrophoresis,  standard proteins, standard proteins,  from Lb fermentum 04BBA19 from Lb fermentum 04BBA19 |
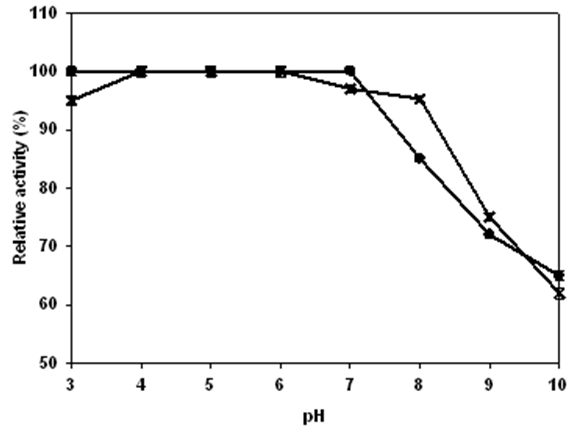 | Figure 8. Effect of pH on the activity (x) and stability  of Lb. fermentum 04BBA19 α-amylase. The data shown are averages of triplicate assays with SD within 10% of mean value of Lb. fermentum 04BBA19 α-amylase. The data shown are averages of triplicate assays with SD within 10% of mean value |
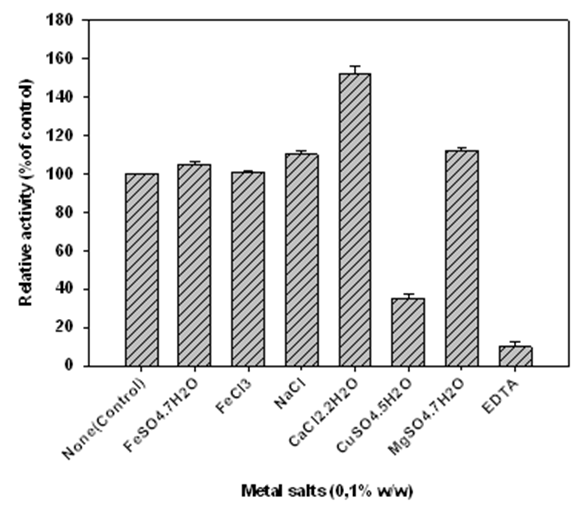 | Figure 9. Effect of metal salts and EDTA on the activity of α-amylase from Lb. fermentum 04BBA19. The data shown are averages of triplicate assays with SD within 10% of mean value |
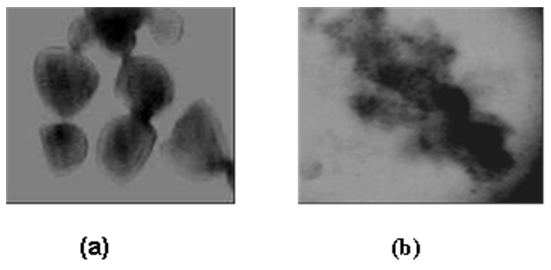 | Figure 10. Photonic micrograph (x600) of untreated raw cassava starch granules (a) and deformed granules (b) after been treated with α-amylase from Lb. fermentum 04BBA19 |
4. Conclusions
- The lactic acid bacterium tested in this study produced amylase with high thermostability, which is not common in lactic bacteria group. The use of this strain in bioprocessing involving amylase production will be advantageous. Because lactic acid bacteria are generally regarded as safe.
ACKNOWLEDGEMENTS
- The authors would like to acknowledge the Teaching Laboratory and the Biotechnology Unit of University of Buea, Cameroon for providing facilities for research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML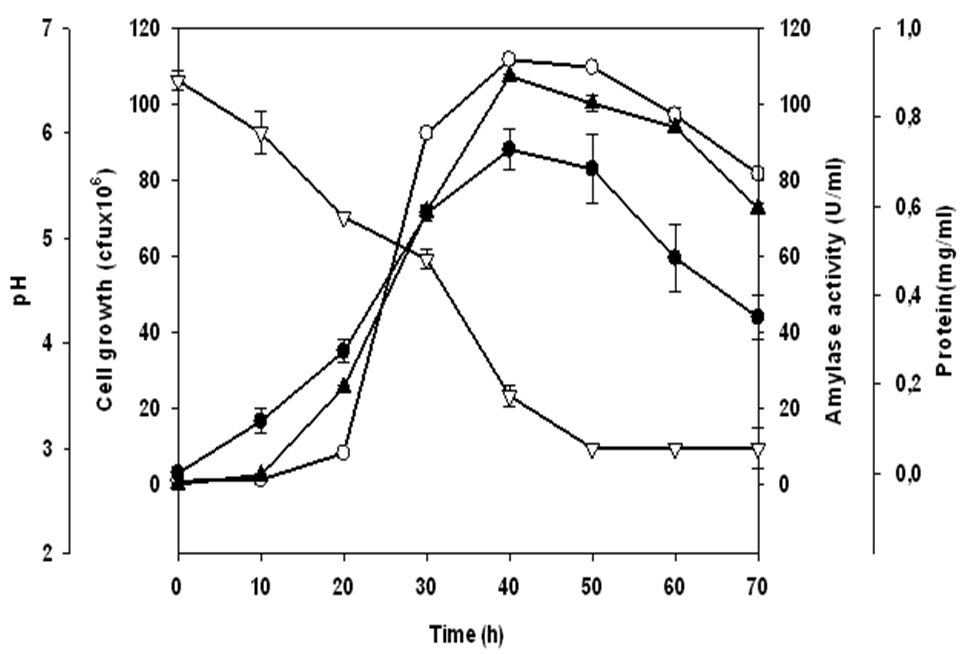
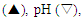 amylase
amylase  and soluble protein
and soluble protein  production by Lactobacillus fermentum 04BBA19 in 1% (w/w) soluble starch medium at 40°C, pH 6.0. The data shown are averages of triplicates assays within 10% of the mean value
production by Lactobacillus fermentum 04BBA19 in 1% (w/w) soluble starch medium at 40°C, pH 6.0. The data shown are averages of triplicates assays within 10% of the mean value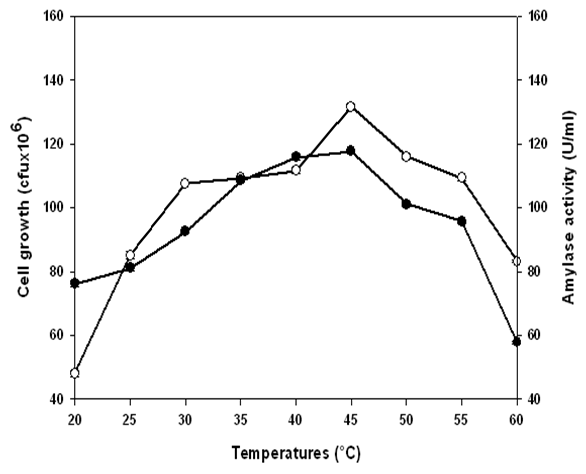
 and α-amylase production
and α-amylase production  by Lb. fermentum 04BBA19 grown in 1% (w/w) soluble starch medium, pH 6.0. The data shown are averages of triplicate assays with SD within 10% of mean value
by Lb. fermentum 04BBA19 grown in 1% (w/w) soluble starch medium, pH 6.0. The data shown are averages of triplicate assays with SD within 10% of mean value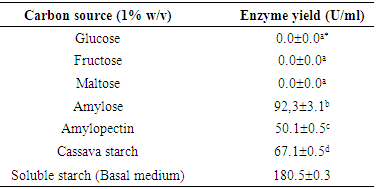
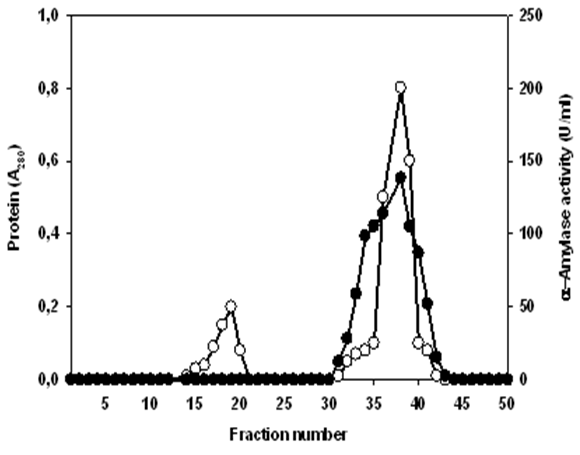
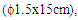 of Lb. fermentum 04BBA19 α-amylase; absorbance at 280 nm
of Lb. fermentum 04BBA19 α-amylase; absorbance at 280 nm  α-amylase activity
α-amylase activity  Column
Column 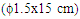 was washed with 0.25 M NaCl to remove impurities; α-amylase from Lb. fermentum 04BBA19 was eluted with 0.05 M Na2CO3 at the flow rate of 3ml/20 min
was washed with 0.25 M NaCl to remove impurities; α-amylase from Lb. fermentum 04BBA19 was eluted with 0.05 M Na2CO3 at the flow rate of 3ml/20 min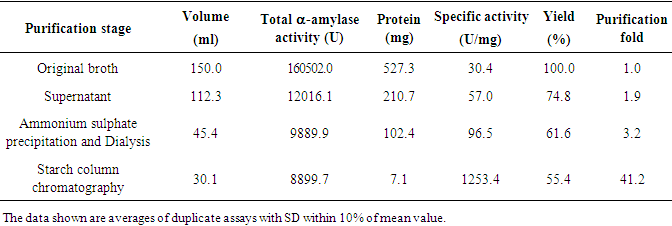
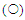 and thermal stability
and thermal stability  of Lb. fermentum 04BBA19 α-amylase. The reaction mixture contained 5 ml substrate (1% w/v soluble starch in 100 mM; phosphate buffer, pH 6.0) and 0.5 ml partial purified enzyme solution. The mixture was incubated for 30 min at various temperatures (30-100°C) under standard enzyme assay condition. The enzyme displayed maximal activity at (60-70°C). For determination of the thermostability of amylase, the enzyme was pre-incubated at optimum pH, for 30 min at temperatures range of 30-100°C. The remaining activity was determined incubating the enzyme at optimum temperature, 60°C for 30 min. The data shown are averages of triplicate assays with SD within 10% of mean value
of Lb. fermentum 04BBA19 α-amylase. The reaction mixture contained 5 ml substrate (1% w/v soluble starch in 100 mM; phosphate buffer, pH 6.0) and 0.5 ml partial purified enzyme solution. The mixture was incubated for 30 min at various temperatures (30-100°C) under standard enzyme assay condition. The enzyme displayed maximal activity at (60-70°C). For determination of the thermostability of amylase, the enzyme was pre-incubated at optimum pH, for 30 min at temperatures range of 30-100°C. The remaining activity was determined incubating the enzyme at optimum temperature, 60°C for 30 min. The data shown are averages of triplicate assays with SD within 10% of mean value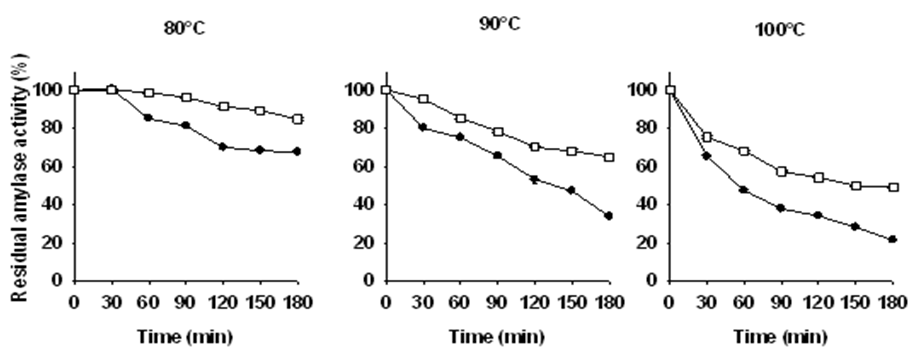
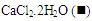 and with 0.1% w/v
and with 0.1% w/v 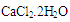
 at different temperatures (80, 90, 100°C). The data shown are averages of triplicate assays with SD within 10% of mean value
at different temperatures (80, 90, 100°C). The data shown are averages of triplicate assays with SD within 10% of mean value