-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(2): 40-46
doi:10.5923/j.microbiology.20160602.03

Antimicrobial Activity of Harmal Plant (Peganum harmala L.) Extract, Ha’il Region, Saudi Arabia
Abdel Moneim E. Sulieman, Ahmed A. Alghamdi, Vajid N. Veettil, Nasir A. Ibrahim
Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present study investigated the antimicrobial activity of Harmal)Peganumharmala)aqueous extracts against two fungi (Aspergillusniger and Peniciliumitalicum) and two Gram negative bacteria (Escerichia coli and Salmonellatyphi). The Harmal methanolic extracts were found to inhibit mycelial radial growth of both fungi. This effect was found to be significant at first day of the experiment as well as the last days. Mycelial fresh and dry weights of both fungi were also greatly reduced with harmal extracts. The higher concentration of Harmal gave the maximum effect which decreased with dilution. The effect on mycelial growth was more pronounced on P.italicum than on A.niger. The effect of Harmal leaves extract on the two bacteria (E.coli and S.typhi) was evaluated by the inhibition zone and dilution methods. A clear zone of inhibition was shown by the extracts against both bacteria, although the inhibition was less against E.coli. The results of the dilution plate method showed that the log number of colonies of both bacteria was highly decreased with Harmal extract; however, S.typhi was more susceptible and greatly affected by the extract.
Keywords: Harmal (Peganumharmala), Antimicrobial activity, Herbalism, Radial growth
Cite this paper: Abdel Moneim E. Sulieman, Ahmed A. Alghamdi, Vajid N. Veettil, Nasir A. Ibrahim, Antimicrobial Activity of Harmal Plant (Peganum harmala L.) Extract, Ha’il Region, Saudi Arabia, Journal of Microbiology Research, Vol. 6 No. 2, 2016, pp. 40-46. doi: 10.5923/j.microbiology.20160602.03.
Article Outline
1. Introduction
- It is estimated that approximately 80% of the developing world's population relies mainly on traditional medicine for primary health care. Where there are still existing systems on the plant plays a vital role in health care. In developed countries, drugs that are extracted from plants are also extremely important for people, currently at least 119 chemicals derived from plant species can be considered as important drugs for human consumption [1].An antimicrobial is a compound that kills or inhibits the growth of microbes such as bacteria. This compound is said to have an antibacterial activity [2]. Medicinal herbs are well documented as a rich source of antimicrobial agents. A wide range of medicinal plant parts are used to extract raw drugs and possess varied medicinal properties [3] [4]. Primitive people learned by trial and error to distinguish useful plants with beneficial effects from the toxic or non-active plants, and also which combinations of the plants or processing methods had to be used to gain consistent and optimal results. This reliance on herbal medicine has proven to be effective in the treatment of long term illness namely diabetes and malaria. Also, it is well known to be preventive measure against some chronic diseases such as pneumonia since it has lesser side effects. In addition, it is a cheaper form of medicine for lots of people [2].Ha’il region is considered to be an important part of the Saudi Arabia’s natural ecosystem since it has a rich biodiversity in terms of fauna and flora. Many plant species belonging to various families and orders were identified in Ha’il area. Natural plants in Ha’il, which are commonly used in different ways as a source of food and herbal medicines and fodder for grazing animals. The most commonly used medicinal plants in Ha’il area include:Pergularia tomentosa L, Peganum harmala L., Cassis and Heliotropium crispum L. Harmal [5] (Peganum harmala L. family Zygophyllaceae) is a perennial, glabrous plant which grows naturally in semi-arid conditions, steppe areas and sandy soils and is native to eastern Mediterranean region. It is a shrub, 0.3-0.8 m tall with short creeping roots, white flowers and round seed capsules carrying more than 50 seeds. The plant is well-known in Iran and is widely distributed and used as a medicinal plant to the Central Asia, North Africa and the Middle East. It has also been introduced in America and Australia. The dried capsules mixed with other ingredients are burnt as a charm against “the evil eye” among Iranians. This plant has many local names such as “Espand” in Iran, “Harmel” in North Africa “African rue” “Mexican rue” or “Turkish rue” in the United States [6]. Various parts of P. harmala including seeds, fruits, root and bark, have been used as folk medicine for a long time in Iran and other countries. Many pharmacological surveys have shown different effects of P. harmala and/or its active alkaloids (particularly harmaline).There were no reports about the effect of Harmal extracts on fungi and bacteria in the literature. Therefore, the present study has been initiated to investigate the antimicrobial activities of the aqueous extracts of harmal (Peganum harmala) plant extract against two fungi (Aspergillus niger and Penicillum italicum) and two bacteria (E. coli and Salmonella typhi).
2. Materials and Methods
2.1. Collection of Samples and Cultures
- Samples of Harmal (Peganum harmala) leaves were obtained from its natural habitat in Aja, Hail city. This medicinal plant was chosen based on local medicinal usage. The leaves were freed from stones, sand and dust, before kept in the labaratory where then washed with distilled water, dried, and milled into fine powder. Aqueous extract was made using methanol alcohol, and different concentrations were prepared as follows: 1.2%, 2.5% and 5%.
 | Figure 1. Harmal plant |
2.2. Effects of Harmal Extracts on Fungal Growth
- The impact of Harmal leaves extract on fungal growth was evaluated through determination of mycelial dry weight and radial growth according to the methods described by Abdel-Rahim et. al. [7] and Abdel-Rahim et al. [8], respectively. For determination of mycelial dry weight, potato dextrose broth (PDB) medium was prepared and then 100 ml dispensed in conical flasks volume (250ml).Serial dilutions were prepared from the extracted solution. The suspension in each flask was sterilized in an autoclave at 121°C (I5-lb/ in12) for 15 minutes, and then allowed to cool down at room temperature before inoculation. Each flask was inoculated by three discs (5.0 mm diameter) taken from an edge of an actively growing culture of the fungus (A. niger) or (P. italicum) grown on solidified PDA (medium.). Inoculated flasks were incubated at room temperature (28°C) for 8 days. After incubation mycelia were collected by filtering the culture through a Whatman No.1 filter paper and fresh weight recorded and then dried at 80°C for 24 hours before being weighed. All treatments were done in triplicates. For determination of Harmal effect on radial growth, potato dextrose agar (PDA) medium was prepared and 100 ml dispensed in conical flasks (250 ml). Then the extract solutions were added to each flask to obtain several dilutions (0.0, 0.50 and 100%). The media were finally sterilized in an autoclave at 121°C (15 Ib/In2) for 15 minutes.After sterilization the solution (medium +extract) of each flask was poured in sterile Petri dishes and left to solidify at room temperature (28-30°C) for 24-40 hour. Each solidified medium was then inoculated centrally by a fungal growth disc cut by a sterile cork-borer (No.5) from the edge of an actively growing culture of the fungus A. niger or the fungus P. italicum grown on PDA. The inoculated Petri dishes were then incubated at room temperature for 8 days and the radial growth was measured every two days. All treatments were done in triplicates.The diameter of disc growth was measured every 48 hours by taking the average of two crossed dimensions for each disc in the Petri dish. The radial growth was then calculated as a percentage from the diameter of the glass Petri dish.
2.3. Effect of Harmal Leaves Extract on Bacterial Growth
- Effect of Harmal extract on the growth of the tested bacteria was determined using two methods: 1- The Cup-Plate agar diffusion (Inhibition zone) methodThis method was achieved by using Nutrient Agar (NA). In this method 2ml of a standardized bacterial cell suspension (10 X 105) of E. coli and S. typhi were thoroughly mixed with 200 ml of sterile molten nutrient agar, and then the medium was distributed into sterile Petri-dishes and was left to solidity at room temperature for 24 hours. Sterile Whatman fiberglass discs (No. 5) were saturated with the extract of Harmal, then allowed to dry and transferred centrally on the surface of the solidified medium in each plate. The plates were then incubated at 33°C for 72 hours and the inhibition zones were measured as described by Barry et al. [9] and Cruickshank et al. [10]. Three replicates were made for each treatment.2- The Dilution Plate Method The effects of the aqueous extracts of Harmal on bacterial growth were investigated by the Dilution Plate Method. Nutrient broth medium batches containing different concentrations of the Harmal extracts were distributed into McCartney bottles. The medium was autoclaved and each bottle was inoculated by 1 ml of the bacterial suspension. Inoculated bottles (three per treatment) were incubated at room temperature and the bacterial growth was measured by the dilution plate method, where 1 ml of the aqueous extract was drawn from each bottle and placed in a tube cooling to 9 ml sterile distilled water and serially diluted.From each dilution 0.1 ml was removed by a sterile pipette and placed on the surface of an already solidified Nutrition Agar (NA) in Petri dishes and spread on that surface, using a sterile glass rod. Inoculated plates were kept at room temperature and the number of colonies was counted at different intervals of time. The number was calculated as the log number of colonies per 1 ml. The logs of the numbers were plotted against time.
3. Results and Discussion
- This study was conducted to investigate the inhibitory effects of Harmal extracts on the growth of two fungi (A. niger and P. italicum) and two Gram- negative bacteria (E. coli and S. typhi). Mycelial radial growth and mycelial dry and fresh weights of both fungi were greatly reduced by Harmal leaves extracts.
3.1. The Antifungal Properties of Harmal Leaves Extract
- To determine the antifungal properties of Harmal plant extracts, the aqueous extracts were tested against mycelial growth (mycelial radial growth and mycelial fresh and dry weights), using PDA and PDB media, respectively. As shown in Fig. 2, extracts of Harmal greatly affected mycelial radial growth of A. niger. This effect was pronounced at the first days but after further incubation the fungus was able to grow better at the lower concentrations and the effect was not significantly different from the control at the 8th day. The effect of Harmal extract on the mycelial weight of the fungus A. niger followed the same pattern of the radial growth, with the higher concentration giving the maximum inhibition that has been detected, and the effect decreased with lower concentration (Fig. 3). However, A. niger was less affected with Harmal extract than P. italicum. Growth of the later fungus was comparatively slower.Figure (4) showed the effect the effect of Harmal extract on radial growth of P. italicum. From the results, it could be seen that the radial growth was greatly reduced with the higher concentration of the Harmal extracts. The effect on P. italicum was more pronounced than on A. niger. More than 50% reduction was recorded for the higher concentration. The result on the effect on the fresh and dry weight (Fig. 5) indicated that both weights were highly reduced with the aqueous extract of Harmal.
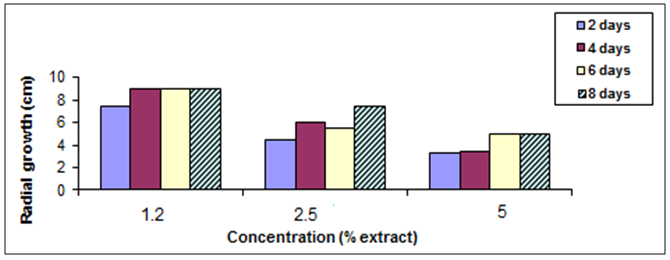 | Figure (2). Effect of different concentration of Harmal effect on radial growth of A. niger |
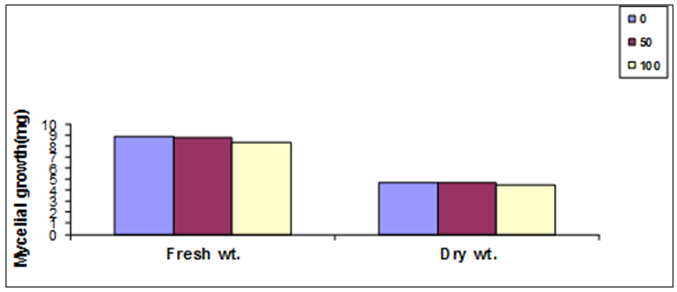 | Figure (3). The effect of different concentration of Harmal extract on mycelial fresh and dry weight of A. niger |
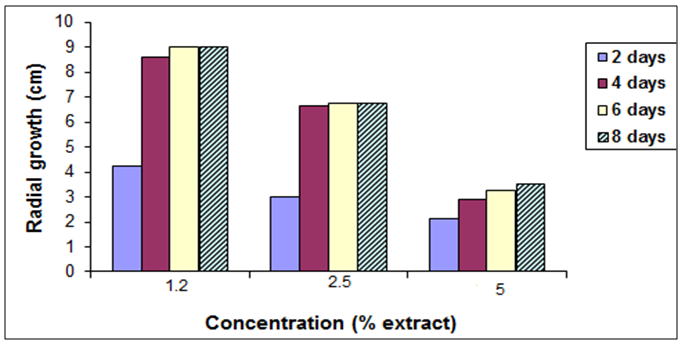 | Figure (4). Effect of different concentration of Harmal extract on radial growth of P. italicum |
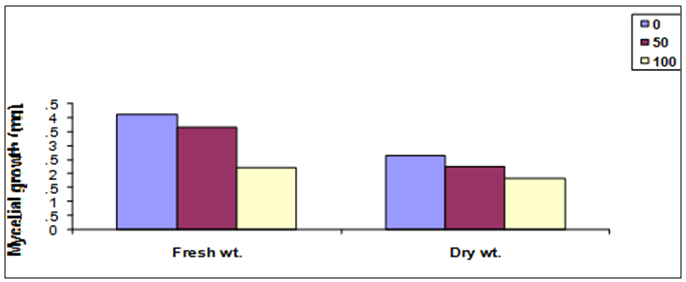 | Figure (5). The effect of different concentration of Harmal extract on mycelial fresh and dry weight of P. italicum |
3.2. Antibacterial Properties of Harmal Leaves Extract
- The present study also investigated the effect of Harmal extract on growth of the two tested bacteria. The two bacteria included E. coli and S. typhi. From the results it is clear that Harmal extract showed a clear inhibition zone of both bacteria. However, the log number of the bacteria E. coli was greatly reduced compared to the control treatment Fig. (6). The log number was gradually decreasing with time. And the higher concentration resulted in higher reduction of the number of bacteria.Figure (7) shows the effect of harmal extract in growth of the bacteria S. typhi. The log number of the bacteria was highly decreased with treatment. The log number was almost zero after 72 hours of incubation with the higher concentration. Comparing the effect of Harmal extract on both bacteria reveals that the later S. typhi was more susceptible and highly affected.Antibacterial activity of spices and other plants have been well documented (Alicia, [20]. Vlietinek et al. [21] screened about 100 medicinal plants, used by traditional healers to treat infections in Rwanda, for their antimicrobial properties. Their study showed that 45% of their plant species were active against bacteria; Staph aureus, 2% against E. coli, 16% against Pseudomonas aerogenosa, and 7% against fungus; Candida albicans. In Sumatra (Indonesia), 114 plant species extracts were assayed for their antibacterial activity [17]. About 82% of the extracts were active against bacteria; Staph aureus, while 35% of them were active against E.coli. On the other hand, Nkuo-Akenji et. al. [22] tested methanol extracts of plant parts commonly used in Cameroon for the treatment of typhoid fever. He found that Cymbopogon citratus leaves, Carica papaya leaves, and Zea mays silk, Emilia coccinea whole plant, Commelina bengalensis leaves, Telfairia occidentalis leaves and Gossypium arboreum whole plant inhibited growth of Salmonella typhi, S. paratyphi and S. typhimurium the microorganism that causes typhoid fever. Moreover, Omoregbe et. al. [23], found that plants extracts-Momordica charantia, Alstonia boonei, and Ocimum bacilicum had some antimicrobial properties against Escherichia coli, Salmonella paratyphi and Shigella dysenterae and suggested their future possible commercial therapeutic use. Ahmed (2004) tested the extracts of 10 Sudanese medicinal plants against gram-positive and gram-negative bacteria as well as on Candida albicans. He found that the effect was greater on Staph aureus followed by the E. coli and Candida albicans. Fenugreek, ginger oil and clove oil were also reported to be effective against Salmoenlla typhimurium [24-26].
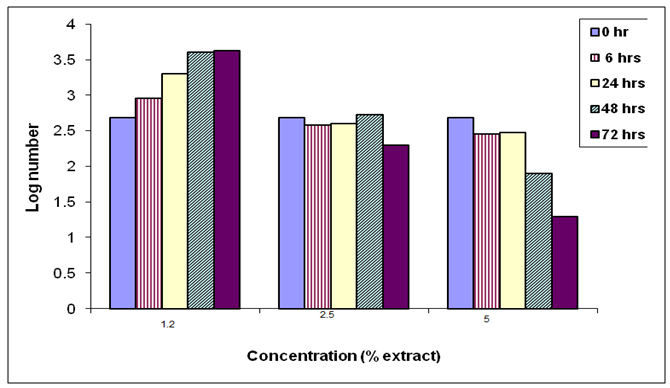 | Figure (6). The effect of the Harmal extracts on log number of the bacterium E. coli |
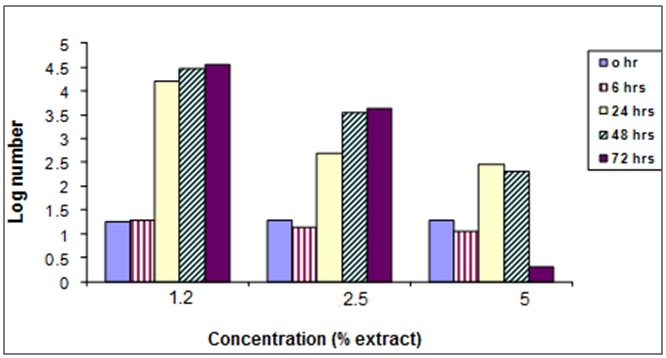 | Figure (7). The effect of Harmal extract on the log number of the bacterium S. typhi |
4. Conclusions
- The study pointed out that aqueous Harmel extract has antifungal and antibacterial against the tested fungi and bacteria. However, the highest inhibitory effect was found against P. italicum. The inhibitory effect against the tested organisms was more effective when using a concentrated extract. According to the current outcomes of this work it could be recommended that Harmal plant extracts can be used as effective antimicrobial agents.
ACKNOWLEDGEMENTS
- The authors express their sincere thanks to the research deanship University of Hail, Saudi Arabia for financing this study (Project No. 01150009).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML