-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(2): 35-39
doi:10.5923/j.microbiology.20160602.02

Antifungal Activity of Infusions from Fresh Oregano, Laurel and Rosemary Leaves and Their Commercial Essential Oils against Acremonium sp.
Racowski I. , Foramiglio V. L. , Teodoro J. A. , Freire V. T.
Laboratory of Microbiology, Faculdade de Tecnologia Termomecanica (FTT), São Bernardo do Campo, São Paulo, Brazil
Correspondence to: Racowski I. , Laboratory of Microbiology, Faculdade de Tecnologia Termomecanica (FTT), São Bernardo do Campo, São Paulo, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Currently, the food industry is constantly challenged by consumers seeking for increasingly healthy foods and less use of synthetic chemical additives such as pesticides or preservatives. In this context, the aim of the present work was to study the antifungal activity of three different commercial essential oils (oregano, laurel and rosemary) in different treatments against phytopathogenic fungus Acremonium sp. and infusions of fresh leaves at 60°C and 100°C in water and 9% gum arabic. The microorganism was naturally isolated from “Debora” type tomato and identified by fungal slide culture. For GIP (Growth Inhibition Percentage) calculation, agar diffusion inhibition analysis was used. With results obtained, it was observed that the three essential oils have effective inhibitory activity against the fungus, and oregano was the most effective, while infusions with and without the addition of gum arabic were not effective. Therefore, essential oils in this study can be used as natural antimicrobials, but in the case of infusions, they have no inhibitory effect on the growth of Acremonium sp.
Keywords: Acremonium sp, Halos of Inhibition, Oregano, Laurel, Rosemary
Cite this paper: Racowski I. , Foramiglio V. L. , Teodoro J. A. , Freire V. T. , Antifungal Activity of Infusions from Fresh Oregano, Laurel and Rosemary Leaves and Their Commercial Essential Oils against Acremonium sp., Journal of Microbiology Research, Vol. 6 No. 2, 2016, pp. 35-39. doi: 10.5923/j.microbiology.20160602.02.
Article Outline
1. Introduction
- Since ancient civilizations, the use of various spices to enhance the flavor of foods and beverages was a common practice. In addition, an increase in the shelf life of these foods was also observed, suggesting that some of these spices would be able to inhibit spoilage and pathogenic microorganisms [1, 4, 8, 24].Currently, due to the ‘clean label’ phenomenon, the food industry faces the need to meet customers increasingly concerned with food security and the use of synthetic additives, particularly preservatives. Similarly, there are also an increasing number of studies on the development of natural antibiotics and herbicides to discourage the use of pesticides demonstrably toxic to humans and the environment [11, 20, 21, 25]. Among these possible new natural antimicrobials, essential oils extracted from traditional spices have become sources of various feasibility studies. These are highly complex, volatile, lipophilic liquid mixtures of intense and characteristic odor, originating from the secondary metabolism of plants, which can be obtained by various physicochemical methods [3, 6, 11, 13].Their constitution varies depending on the species, age and plant part, soil and climate of the region, susceptibility to pathogens and production method, but have commonly high contents of terpenes, alcohols, aldehydes, ketones, phenols and esters, and generally, a single substance appears much larger content than the others, being responsible for the characteristic flavor and odor of the plant [17, 20, 24, 31]. The genus Acremonium sp., formerly known as Cephalosporium sp., is described as composed of saprophytic species found with other fungi in foods such as apple, pear, maize, tomato, coffee, celery, sorghum and rice. It is also commonly found in the air or soil. However, it is also considered one of the most aggressive pathogens to maize (Zea mays L.), which may cause seed rot, low germination level, seedling death, stalk rot, and decreased plant growth, yield and productivity, causing serious economic losses [5, 9, 16, 19, 30]. Based on the above, this aims of this study were two: the first was to evaluate the antimicrobial activity of commercial essential oils of Oregano (Origanum vulgare), Laurel (Laurus nobilis) and Rosemary (Rosmarinus officinalis) on microorganism Acremonium sp. and the second was to determine the antimicrobial activity of infusions of fresh leaves from these spices in water and in 9% gum arabic at 60°C and 100°C.
2. Method and Materials
2.1. Materials
- Analyses were developed in the Laboratory of Microbiology of the Termomecanica Faculty of Technology between August and November 2015. Commercial essential oils extracted from rosemary, oregano and laurel were provided by Symrise® company. Fresh leaves of three vegetables were acquired in the trade of the city of São Bernardo do Campo. Microorganism Acremonium sp. was isolated from “Debora” type tomatoes.
2.2. Isolation of the Microorganism of Interest
- In order to obtain a pure fungal colony for inhibition analysis, isolation and replating of this microorganism were performed according to recommendations of Moraes, Paes and Holanda [15], with modifications as Racowski et al propose [20]. For this, “Debora” type tomato samples were left outdoors in an open glass container exposed to sunlight at room temperature until clear signs of fungal contamination were observed. Later, hyphae of the microorganism formed on the food surface were taken with the aid of an inoculation loop. Points where the colony appeared single and uniform were chosen to avoid the collection of more than one mold species. Hyphae were then seeded in Petri dishes containing solid PDA medium (Potato Dextrose Agar) in the presence of chloramphenicol antibiotic. After inoculation, plates were stored in BOD incubator for 5 days at 28°C. After growth, replating and incubation were performed under the same conditions in a new PDA plate by smear technique, which was sufficient for the isolation of a single fungus species. The replating procedure was repeated every week in order to have always fresh microorganism.
2.3. Identification of the Filamentous Fungus by Fungal Slide Culture
- For fungal identification, fungal slide culture analysis was performed according to Ribeiro & Soares [21], with modifications proposed by the identification key of Taniwaki & Silva [29] apud Pitt & Hocking (1997): scraps of solidified PDA medium were placed on a glass slide, placed on the top of a blade holder. This set was placed in an empty Petri dish with only a small amount of distilled water at the bottom on a filter paper to provide moisture for the growth of the fungus. On the side walls of the solidified medium, the colony of isolated fungus was inoculated with the aid of a disposable inoculation loop, being covered with a coverslip. The plate was closed and placed in an oven at 28°C for 3 days. After fungal growth, the coverslip was removed and the fungus was analyzed in common bench microscope. Along with the macromorphological characteristics of the plate, the micromorphology observed in different magnifications was compared with Mycology Atlas up to the visual identification of the species.
2.4. Production of the Infusions
- Infusions were performed using fresh oregano, rosemary and laurel leaves. For this, methodology proposed by Lima, Melo and Lima [14]; Katalinic et al [13]; and Su et al [27] was used, where 10 g of fresh leaves were immersed in 100 mL of distilled water when it reached the desired temperature. Infusions were carried out at temperatures of 60 and 100°C. The addition of leaves occurred only when the water temperature reached the two different temperatures and leaves remained in contact with hot water for 15 minutes. Infusions were cooled to room temperature, filtered and used in inhibition tests. In infusion prepared with gum arabic, the procedure was exactly the same as described above but replacing distilled water by a 9% gum arabic solution at both temperatures.
2.5. Antifungal Activity Test
- Oils and infusions were diluted in distilled water at a ratio of 0 (control), 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100%, all of them using distilled water as diluent.Analysis was performed according to Hillen et al [12], with modifications suggested by Venturoso et al [31] and Diniz et al [8]: a sample of 150 μL of each dilution was pipetted and spread with the aid of a Drigalsky loop on the surface of a different Petri dish containing sterile PDA medium with chloramphenicol. After the complete treatment absorption with the aid of a stick stopper flamed in Bunsen burner, 3 wells were performed per plate, with 5 mm in diameter. Using disposable inoculation loops, a microorganism replating was inoculated into the inner walls of each well. For purposes of control, a plate was prepared, inoculated and perforated similarly for each microorganism without, however, receiving any added antimicrobial agent. The fungal replating used had at least 4 and at most 7 days of age. After properly inoculated, plates were inverted and stored in an oven at 28°C and observed for 48h. The diameters of colonies around each well were measured at that time. The measures were taken based on the largest diameter of each colony formed around each well, and then a second measure was diametrically opposed to the first. These measures were used to obtain the average and the standard deviation that were used to calculate the Growth Inhibition Percentage (GIP). The principle of inhibition analysis is to check how much the fungal colony can develop from where it was inoculated until it was inhibited by the antimicrobial agent that is disposed on the plate surface. The Growth Inhibition Percentage (GIP) represents how much a colony had its growth inhibited compared to control, in this case, the plates where there was no addition of inhibitor, which facilitates the interpretation of results. The GIP calculation follows the formula of equation (1), according to Diniz et al [8] apud Edginton et al (1971):
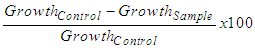 | (1) |
2.6. Statistical Analysis
- All experiments were performed in triplicate. Data were analyzed using SPSS v.13.0 software; Chicago, Inc, applying ANOVA and Tukey's test (5% significance level).
3. Results and Discussion
3.1. Isolation and Identification of the Microorganism
- The apparent mycelium in “Debora” type tomato took about 15 days to form. The mycelium color throughout the contamination extent was whitish with cottony characteristic, i.e. white velvety aspect. Under analysis, the fruiting body in the image (Figure 1) shows the appearance of elongated elliptical structures, typical characteristic of the fungus Acremonium sp., as described and shown by Ellis & Hermanis [9]. The macromorphology found is also consistent with that described by Christensen et al [5]: The species of Acremonium have the morphology of the colonies usually very similar to each other: white, cottony and rapid growth.
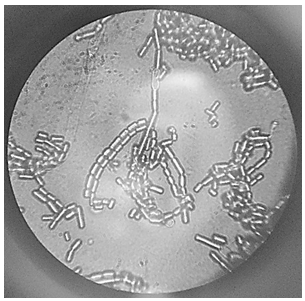 | Figure 1. Fruiting body of fungus Acremonium sp. with magnification of 1000x in optical microscope |
3.2. Antifungal Activity and Growth Inhibition Percentage (GIP) Calculation
- The measurements obtained and the corresponding GIP values for the solutions of the three essential oils are shown in Table 1. The solutions of infusions at 60°C and 100°C and with gum arabic were not computed because they did not present microbial inhibition.The reason of using gum arabic was because most compounds that act as antimicrobial agents in essential oils are hydrophobic, and in an attempt to collect them in infusions and check the antimicrobial power of these substances, gum arabic was used. According to Pasquel [18]; Rottava [22] and Gabas & Cavalcanti [10], this gum has hydrophilic and hydrophobic characteristics, which may help in dissolving the antimicrobial agent at the time of heating when performing the infusion, and remain in suspension as dilutions from 10 to 100%, since these were performed in distilled water. It is noteworthy that according to Caleguer & Benassi [2], the maximum concentration so that there is no change in viscosity of a solution containing gum arabic is up to 10%. In our case, we chose to use a 9% solution, since otherwise we would have difficulty in handling this solution in further analysis.The obtained measures and the correspondently GIP values for the three solutions of the three essential oils are at Table 1.
|
4. Conclusions
- Essential oregano, rosemary and laurel oils have inhibition activity against the growth of Acremonium sp. when diluted in distilled water. The most efficient oil in the inhibition of Acremonium sp. was oregano oil, since even at concentration of 20%, it was able to fully inhibit the growth of Acremonium sp. The least effective oils tested was rosemary, since even pure, it was able to inhibit the growth of Acremonium only by 44%. Infusions in water of oregano, rosemary and laurel leaves produced at 60°C and 100°C showed no inhibition activity against the growth of Acremonium sp.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML