-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(1): 8-13
doi:10.5923/j.microbiology.20160601.02

Microbiological Characteristics and Sensory Evaluation of White Cheese Produced by Using Camel Milk and Mixture of Camel and Cow Milk
Abdel Moneim E. Sulieman1, 2, Salma M. Siddig2, Zakaria A. Salih2
1Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia
2Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The objective of the present study was to assess the quality of white cheese produced using raw camel milk, and a mixture of camel milk and cow milk (1:1). Raw milk samples obtained from Wad Medani local markets were subjected to microbiological analysis before production of cheese. Results showed that raw camel and cow milk samples contained 1x104 and 1x106c.f.u/ml total bacterial count and devoid from any coliforms or Staphylococcus aureus cells. Cheeses were prepared by acidification using10% citric acid and by adding lactic acid bacteria (LAB) starter culture (5%). The total bacterial count were 15x106, 9x105 and 11x105 (c.f.u/ml), respectively, while the yeast and moulds counts were 2x103, 6x102 and 8x102 (c.f.u/ml), and the lactic acid bacterial were 1.5x108 3x105and 4x105c.f.u/ml in PCM, MCCM1 and MCCM2, respectively, in cheeses produced from pure camel milk (PCM), mixture of camel milk and cow milk coagulated with acidification (MCCM1) and mixture of camel milk and cow milk coagulated with lactic acid bacterial culture (MCCM2). On the other hand, all samples contained coliform bacterial which ranged between 2.5x105 and 1x106c.f.u/ml, and devoid from any Staphylococcus aureus cells except in the MCCM2 which contained 2x105c.f.u/ml. Mixing of camel milk with cow milk improved the microbiological and sensory quality of the resultant cheese. The sensory evaluation indicated acceptance of all cheeses with preference to those prepared with lactic acid bacteria.
Keywords: Lactic acid bacteria, Yeast, Mould, Coliform, Safety
Cite this paper: Abdel Moneim E. Sulieman, Salma M. Siddig, Zakaria A. Salih, Microbiological Characteristics and Sensory Evaluation of White Cheese Produced by Using Camel Milk and Mixture of Camel and Cow Milk, Journal of Microbiology Research, Vol. 6 No. 1, 2016, pp. 8-13. doi: 10.5923/j.microbiology.20160601.02.
Article Outline
1. Introduction
- Camels are a good source of milk and meat, besides, it is used by many nations and are used for other purposes such as transportation and sport racing. Camel milk has an important role in human nutrition particularly in the hot regions and arid countries. This milk contains all the essential nutrients found in bovine milk [1].Most camel milk production is consumed locally by families and their animals, and does not reach the urban markets because most of the camel herds are located in the arid and desert areas which are far from the commercial markets in Sudan. However, sometimes few amounts of camel milk is processed traditionally into a fermented product called Garris which is consumed locally. Cheese making technology aims to preserve milk so that consumption can be postponed for periods from few days to several months. The production of cheese, like many other food preservation processes allows the nutritional and economic value of a food material. It is employed in many places to control the spoiling of milk where surplus amounts of milk are converted to products or spoiled. The original aim of cheese manufacture was extending the shelf life and conserves the nutritious components of milk. This is achieved either by acid production and/or dehydration. Production of lactic acid by the starter flora during cheese manufacture results in a decrease in the pH of the milk and this, in combination with cooking and stirring, promotes syneresis of the curd and expulsion of whey.It has been reported that cheese making from camel milk is a difficult process because camel milk takes a longer coagulation time and the coagulum is weak [2-4]. Camel milk has poor rennet ability because of differences in availability of κ-casein, camel milk has more large casein micelles than doe’s cow milk. Another reason is that camel milk contains abnormally low milk solids and its cheese processing ability is poor.Milk used for cheese making must be of an acceptable microbiological quality, free from pathogens, antibiotics and inhibitors, and has a total plate count of less than104 cfu/ml. Benkerroum [5] found that the camel milk cheese has an acceptable hygienic quality, and he attributed this to the absence of faecal coliforms, faecal streptococci, Salmonella spp. and Clostridial spores. In addition, the inherent antimicrobial activity of camel milk could concur with the highly competitive nature of the lactic acid bacteria of the starter culture to limit the growth of undesirable microorganisms during the fermentation. Since the production white cheese from pure camel milk is a difficult process, the present study was initiated to produce white cheese from camel milk and a mixture of camel milk and cow milk (1:1) by direct acidification and starter culture of lactic acid bacteria (LAB) and to assess the microbiological quality as well as sensory evaluation of these cheeses.
2. Materials and Methods
2.1. Materials
- Fresh whole camel and cow milk were collected using 1000 mL, autoclave able plastic containers which have been pre-sterilized at 120 C for 15 min and kept ready for milk collection from local market at Wad Medani, central Sudan. A box containing ice was used to provide cold storage during transport to to the laboratory of the Department of Food Science and Technology, University of Gezira pending analyses and production of cheese.All chemicals used were analytical grade.
2.2. Cheese Preparation
- Eight liters of camel milk and 4 liters cow milk were taken in stainless steel containers and heated to 65°C for 30 minutes. The temperature of milk was brought down to 40°C. Three types of cheese were prepared (Fig.1): the first type was prepared with addition of 10% citric acid to pure camel (PCM), the second type was prepared with addition of 10% citric acid to a mixture of camel milk and cow milk (1:1) (MCCM1), and the third type was prepared with the addition of 5% starter culture consisting from lactic acid bacteriato a mixture of camel and cow milk (1:1) (MCCM2).
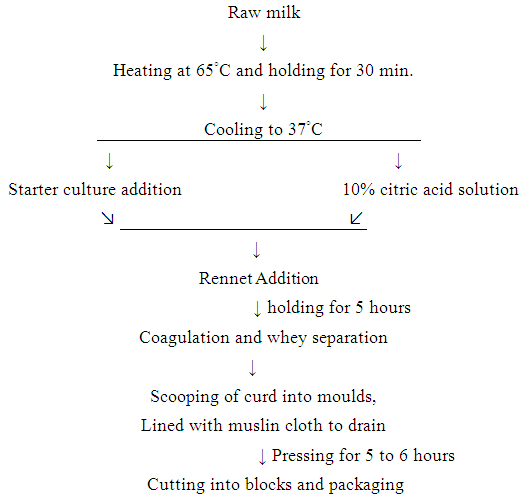 | Figure 1. Cheese production flow diagram |
2.3. Methods
2.3.1. Preparation of Serial Dilution
- Ten gram samples of either raw milk or cheese were homogenized with 90 ml of distilled water by shaking for several minutes, from this suspension. 1 ml was taken from the dilution and transferred to another tube to make serial dilution up to10-6.
2.3.2. Total Bacterial Count
- The total viable count per ml of sample was obtained by pour- plating suitable dilution in triplicates on plate count ager (Oxoid) following the method of APHA [6]. Incubation was accomplished at 37°C for 48 hours. Plates containing between 30-300 colonies were counted as colony forming units (c.f.u) per ml of the sample.
2.3.3. Yeasts and Moulds Count
- Yeasts and moulds were enumerated according to Marshall [7] using Potato Dextrose Agar (PDA). The plates were incubated at 25°C for 3-5 days, plates containing between 30 and 300 colonies were counted as colony forming units (c.f.u /ml).
2.3.4. Lactic Acid Bacterial Count
- Lactic acid bacterial count was determined according to the method described by Kiss [8] using MRS media after anaerobic incubation at 37°C for 48 hours the different types of colonies were counted. Plates containing between 30 to300 colonies were counted a colony forming units (c.f.u) per ml of samples.
2.3.5. Coliform Bacterial Count
- Coliform bacterial count was determined according to Marshall [9] using MacConkey broth. The tubes were incubated at 37°C for 48 hours. Positive tubes gave gas in Durtam tubes. Then the positive tubes were sub cultured into EC broth medium and then incubated at 44°C for 24 hours to determine the coliform bacteria, the tubes showing any amount of gas production were considered positive.
2.3.6. Staphylococcus Aureus Count
- From suitable dilution, 0.1 ml was plated onto Baird Parker Agar media and inoculums were distributed evenly using sterile glass rod. The plates were then incubated at 37°C for 24-48 hours and the counts were presented as colony forming units per gram (c.f.u/ml).
2.3.7. Sensory Evaluation and Statistical Analysis
- Sensory characteristics were determined by 20 untrained panelists for judging the quality of the various cheeses using 9-point hedonic scale (1: extremely bad, 9: Excellent) to assess appearance, flavour, texture and overall acceptability.Statistical analysis of data was subjected to analysis of variance by using Statistical Package for the Social Sciences (SPSS) program version 20.
3. Results and Discussion
3.1. Microbiological Characteristics of Raw Camel and Cow Milk
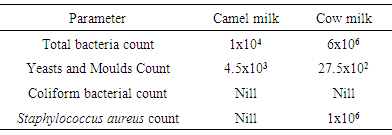
- The data recorded in Table (1) present the microbiological characteristics of the raw camel milk and raw cow milk. The total bacteria and yeasts and moulds counts were 1x104 and 4.5x103c.f.u/ml, respectively for raw camel milk. However, these samples were devoid from any coliforms and Staphylococcus aureus cells. These values are lower than those reported by Benkerroum [5] who found that raw camel milk contained 6.2 x107, 3.8 x104 and 1.1x104c.f.u/ml of total bacteria count, yeasts and moulds count and Staphylococcus aureus, respectively. On the other hand, cows’ raw milk contained 6 x 106, 27.5 x 102 and 1x106c.f.u/ml total bacteria, yeasts and moulds and Staphylococcus aureus, respectively, and it did not contain coliform bacteria. These results disagree with those reported by Ali [10] who found that the raw cow milk samples collected from different sources in Khartoum contained 9.88x106, 9.63 x 105, 5.43 x 104 and 1.20 x 106c.f.u/ml total bacteria count, yeasts and moulds count coliform bacterial and Staphylococcus aureus, respectively. It has been reported that camel milk has an antimicrobial effect against Gram positive and Gram negative bacteria Staphylococcus aureus. The inhibitory action of camel milk against S. aureus and E. coli might be attributed to the presence of lactoperoxidase, hydrogen peroxide, lactoferrin and immunoglobulins and lysozyme [11] [12]. In addition to the absence of harmful pathogenic bacteria so itis safe for human consumption.
|
3.2. Microbiological Characteristics of the Cheese
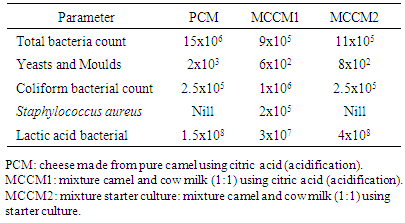
- The microbiological characteristics of the various cheese types are presented in Table (2). The results showed that the total bacteria count of pure camel cheese (PCM), mixture of camel and cow milk (MCCM1) coagulated with citric acid and mixture of camel milk and cow milk coagulated with starter culture (MCCM2) were 15x106, 9x105 and 11x105c.f.u\ ml, respectively, these results are in close agreement to the values determined by Abdl Whap et al [13] who stated that total bacteria count for pure camel fresh milk cheese was 15.82 ×105(c.f.u/g), moreover, Shahein et al. [14] found relatively similar values (1 x 106 c.f.u/g and 5 x 106(c.f.u/g) for pure camel milk cheese and 5 x 106 (c.f.u/g) for cheese prepared from a mixture camel (60%) and cow milk (40%), respectively. The relatively higher bacterial load in the tested samples might be due to either insufficient heating of milk and/or the lower initial survival rate of bacteria in cheese.Table (2) also shows that yeasts and moulds count was 2 x 103, 6 x 102 and 8x102 (c.f.u/g), respectively, in PCM, MCCM1 and MCCM2, respectively. However, these values were lower than those reported by Abdl Whap et al. [13] which was 4.02×105 c.f.u/g) for pure camel milk cheese. Yeasts and moulds are considered as spoilage organisms resulting in flavour and textural deteriorate on including softening, discoloration and slime formation [15]. Because yeasts can grow under conditions of high salt or sugar content, they can cause the spoilage of certain foods in which bacteria would not ordinarily grow. Examples are honey, jellies, maple syrup, and sweetened condensed milk. Foods produced by the bacterial fermentation process such as pickles and sauerkraut, can also be spoiled by yeasts which interfere with the normal fermentative process. Although moulds are important to the food industry such as their many contributions in flavor and color they add to cheeses, they can also cause problems in foods through the toxin molds they (mycotoxins). The microbiological analysis also showed that all produced cheese samples contained coliforms in high counts. PCM, MCCM1 and MCCM2 contained 2.5x105, 1x106 and 2.5x105 c.f.u/g coliforms, respectively, however these values were lower than the counts 9.08×105 (c.f.u/g) reported by Abdl Whap et al. [13] for pure camel milk cheese. Presence of coliforms in these levels might be attributed to the lack of proper handling and hence contamination by microorganisms, as most of camel owners practiced less hygiene during milking and storage of their milk. The colifoms counts determined in the presence study exceeded the cheese standard of Canada which state that the colifom must not exceed 102 c.f.u/g and 5.0x 102 c.f.u/g in pasteurized cheese and raw milk cheese, respectively.Results of the microbiological analysis showed that all samples were devoid from any Staphylococcus aureus cells except in the MCCM2 which contained 2 x 105 c.f.u/g. It has been reported that camel milk has an antimicrobial effect against Gram positive and Gram negative bacteria Staphylococcus aureus. The Staphylococcus aureus counts determined in the presence study exceeded the cheese standard of Canada which state that the Staphylococcus aureus must be less than 103 c.f.u/g in raw milk cheese. Presence of harmful bacteria such as coliforms and Staphylococcus aureus may create health risks to the cheese consumers. Generally, presence of antibacterial substances such as lactoperoxidase, hydrogen peroxide and lysozyme of camel milk can reduce the counts of pathogens [11], in addition, improvement of hygienic measures are advised during processing and handling of camel milk cheese. In general, since the raw milk used for cheese making had been pasteurized, the higher microbial load of various cheeses could be attributed to improper hygienic practices during processing and handling, and could indicated post contamination not from the initial, for example: water quality, mould, muslin cloth Improvement of the unhygienic environment of cheese production plant will lead to production conditions of cheese with an acceptable microbiological quality.
|
3.3. Sensory Evaluation of the Cheeses
- Sensory characteristics of cheeses are considered one the most important attributes determining the consumers’ choice. Before and during ingestion itself, the consumer can perceive several sensory features of cheese, which are generally grouped under appearance, flavour and texture. All such attributes determine the eating quality of cheeses and consequently their acceptability. There is a wide diversity of cheese types worldwide, each one with a unique sensory profile. It reflects the characteristics of the milk feedstock, the cheese making conditions and the physical and chemical changes throughout ripening [19]. The results of sensory evaluation of various cheeses are shown in Fig. 2-4. The results show that the cheese samples prepared by using starter culture were more preferred on the basis of appearance, flavour and texture as compared to cheeses obtained by direct acidification using citric acid. However, all cheeses were accepted by the panelists.
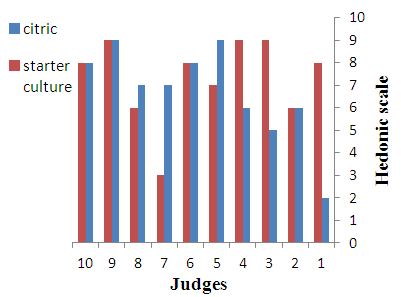 | Figure 2. Appearnace of Cheeses |
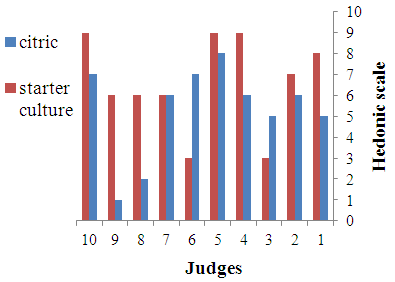 | Figure 3. Taste of cheeses |
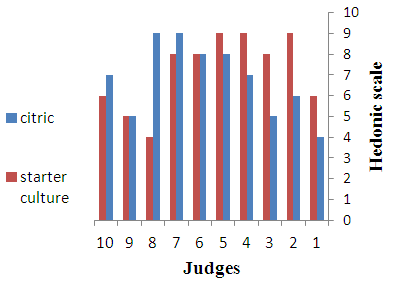 | Figure 4. Texture of Cheeses |
4. Conclusions
- The present study confirmed the possibility of production of cheese, from camel milk using a mixture of camel milk and cow milk coagulated with citric acid and lactic acid bacteria starter culture with an acceptable sensory quality. However, the microbiological quality of cheeses indicated higher bacterial contamination than the raw milk used which imply the unhygienic conditions during processing of cheese. The safety of consuming these cheeses could be improved by using proper hygienic practices during processing and handling of them.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the administration, staff members and technicians of the Department of Food Science and Technology, Gezira University for the encouragement and assistance in conducting this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML