-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2016; 6(1): 1-7
doi:10.5923/j.microbiology.20160601.01

In vitro Activity of Syzygium aromaticum against Food Spoilage Fungi and Its Potential Use as an Antiradical Agent
Sokamte T. A. 1, Jazet D. P. M. 2, Tatsadjieu N. L. 3
1Department of Food Science and Nutrition, National School of Agro-Industrial Sciences, University of Ngaoundere, Ngaoundere, Cameroon
2Department of Biochemistry, Faculty of Sciences, University of Douala, Douala, Cameroon
3Department of Food Engineering and Quality Control, University Institute of Technology, University of Ngaoundere, Ngaoundere, Cameroon
Correspondence to: Tatsadjieu N. L. , Department of Food Engineering and Quality Control, University Institute of Technology, University of Ngaoundere, Ngaoundere, Cameroon.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the present study was to investigate the essential oil of Syzygium aromaticum from Cameroon for its chemical composition, antiradical and antifungal activities against some common fungi causing spoilage of stored food products. The essential oil, obtained by hydrodistillation of dry fruits, was analyzed by GC and GC/MS. The main components of the oil were eugenol (81.9%), α - elemene (7.7%) and δ - Cadinene (10.2%). Determination of antiradical activity of the oil was studied by the DPPH (diphenyl picrylhydrazyl) method. The antiradical activity of Syzygium aromaticum essential oil (SC50 = 23.17 mg/L) was higher than that of butylated hydroxy toluene (BHT), which was used as the reference compound (SC50 = 65.03 mg/L). The evaluation of the antifungal activity of the essential oil of S. aromaticum by the incorporation technique showed a strong antifungal activity with minimum inhibitory concentration (MIC) of 300 ppm against A. niger and A. carbonarius, 400 ppm against A. flavus, A. versicolor and F. oxysporium, and finally 500 ppm against A. fumigatus. A. fumigatus was the most resistant fungal strain to the essential oil of S. aromaticum. Results obtained in the present study indicate the possibility of exploiting Syzygium aromaticum essential oil to fight against strains of A. niger and A. carbonarius, A. flavus, A. versicolor and F. oxysporium responsible for biodeterioration of stored food products.
Keywords: Essential oil, S. aromaticum, Chemical composition, Antioxidant activity, Antifungal activity
Cite this paper: Sokamte T. A. , Jazet D. P. M. , Tatsadjieu N. L. , In vitro Activity of Syzygium aromaticum against Food Spoilage Fungi and Its Potential Use as an Antiradical Agent, Journal of Microbiology Research, Vol. 6 No. 1, 2016, pp. 1-7. doi: 10.5923/j.microbiology.20160601.01.
Article Outline
1. Introduction
- The presence of molds in foodstuffs can lead to deterioration, with major consequences being the reduction of food value (deterioration of the nutrients) and the reduction of organoleptic qualities (color, taste, odor, texture). Thus, it is estimated that the uncontrolled growth of molds in food stuffs leads to 5-10% loss of food harvests in the world annually [1]. Under favorable conditions of temperature, moisture, pH and composition of substrate, a great number of molds species are able during their development on many foodstuffs, to synthesize and excrete toxic secondary metabolites known as mycotoxins. Among a hundred mycotoxins identified presently, about thirty is truly significant for human and animal health because of their contamination frequency or their toxicity [2]. The principal fungal species that produce mycotoxins belong to the genus Aspergillus, Penicillium and Fusarium [3].In addition, the free radicals produced naturally by oxidation of food substances in free radical chain reactions, constitute a major problem in the conservation and preservation of manufactured food. These free radicals can initiate in consumers the oxidation and hence destruction of many organic molecules of biological importance and they are the origin of many diseases [4].In order to control the damage due to molds and the oxidation of food substances, food industries generally make use of chemical fungicides (benzoic propionates, acids and their salts) and synthetic antioxidants such as butyl hydroxytoluene (BHT) and butyl hydroxyanisole (BHA). However, although endowed with a great efficacy, the use of these synthetic chemical molecules in food preservation is increasingly being reduced in food industries worldwide, because of the enormous negative effects associated to their use such as risk of cancer [5]. On account of these negative effects of synthetic chemical molecules on the health of man, animals and quality of food, the research of alternatives methods of natural origin is very important for the control of these pathogenic molds, as well as free radicals in foodstuffs [6]. Essential oils and their components currently employed as food flavorings are also known to have antimicrobial and antioxidant properties. Thus, they could be used as food preservatives, since a majority is classified as "safe" [7]. The use of essential oils as bio-conservatives became of great interest, especially for foodstuffs owing to the fact that consumers seek naturally preserved food [8]. These last years, much work has been done on the valorization of the properties of essential oils as natural preservatives [6].In Cameroon, Syzygium aromaticum is largely exploited for its dry fruits, as spice and in traditional medicine (analgesics dental, antinevralgic, disinfectants, aromatic, stimulative, stomachic) [9]. Work carried out on the dry fruits of this plant showed that it was endowed with excellent antibacterial and antioxidant properties [10, 11]. As part of main study aimed on screening antifungal extracts of native plants from Cameroon, we evaluated the antifungal activity of plant used traditionally for several purposes including antimicrobial effects. The aim of the present study was to determine the effects of essential oil of Syzygium aromaticum fruits on the growth of A. niger, A. carbonarius, A. flavus, A. versicolor, A. fumigatus and F. oxysporium and to determine its antiradical activity. Results obtained might yield significant information as to whether essential oil of this plant can be used as food preservatives.
2. Materials and Methods
2.1. Fungal Strains
- The fungal species used in this work were: a strain of Aspergillus niger, Aspergillus carbonarius, Aspergillus fumigatus, Aspergillus versicolor, Aspergillus flavus and a strain of Fusarium oxysporium. These strains were selected for their high frequency to contaminate foodstuffs and pathogenicity. All strains were offered by the Microbiology Laboratory of the National High School of Agro-Industrial Sciences, University of Ngaoundere, Cameroon.
2.2. Plant Material and Extraction of Essential Oil
- The plant material used in this study consisted of the dry fruits of S. aromaticum collected in Bafoussam (West Cameroon) in July (2012). These plants were identified at the National Herbarium of Cameroon. The extraction of the essential oil was carried out by hydrodistillation with Clevenger apparatus. The recovered oil was stored at 4°C until use [12]. The extraction yields were calculated in percentage (v/w) relative to the starting plant material.
2.3. Chemical Analysis of Essential Oil
- Essential oils obtained were analyzed by gas chromatography (GC) and gas chromatography coupled with mass spectrometry (GC/MS).
2.3.1. Gas Chromatography
- The oil was analyzed on a Varian CP-3380 GC with flame ionisation detector fitted with a fused silica capillary column (30 m x 0.25 mm coated with DB5, film thickness 0.25 m); temperature program 50°- 200°C at 5°C/min, injector temperature 200°C, detector temperature 200°C, carrier gas N2 1ml/min. The linear retention indices of the components were determined relatively to the retention times of a series of n-alkanes and the percentage compositions were obtained from electronic integration measurements without taking into account relative response factors.
2.3.2. Gas Chromatography/Mass Spectrometry
- GC/MS analyses were performed using a Hewlett-Packard apparatus equipped with an HP1 fused silica column (30 m x 0.25 mm, film thickness 0.25 m) and interfaced with a quadrupole detector (GC quadrupole MS system, model 5970). Column temperature was programmed from 70°-200°C at 10°C/min; injector temperature was 200°C. Helium was used as carrier gas at a flow rate of 0.6 ml/min. The mass spectrometer was operated at 70eV.
2.3.3. Identification of the Components by Their Retention Indices
- Identification of the constituents was assigned on the basis of comparison of their retention indices and their mass spectra with those given in literature [13].
2.4. Antifungal Activity
- Antifungal assay was performed using the agar disc diffusion [12]. Sabouraud dextrose agar (SDA) medium with different concentrations of essential oils (100 ppm, 200 ppm, 300 ppm up to 1000 ppm) were prepared by adding the appropriate quantity of essential oil to the melted medium, followed by manual rotation of the Erlenmeyer flask to disperse the oil in the medium. About 20 mL of the medium was poured into glass Petri–dishes (9 cm x 1.5 cm). Each Petri–dish was inoculated at the center with a mycelial disc (6 mm diameter) taken at the periphery of a fungal strain colony grown on SDA for 48 h. Control plates (without essential oil) were inoculated following the same procedure. Plates were incubated at 25 ± 2°C and the colony diameter was recorded each day. Minimal inhibitory concentration (MIC) was defined as the lowest concentration of essential oil in which no growth occurred. For each concentration, 3 tests were carried out.
2.5. Evaluation of Antiradical Activity
- The antiradical activity was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free stable radical scavenger [14], which was dissolved in ethanol to give a 100 μM solution. To 2 mL of the ethanolic solution of DPPH was added 100 μL of a methanolic solution of an antioxidant reference (BHT) at different concentrations. The oil was tested using the same method. The control without antioxidant was represented by the DPPH ethanolic solution containing 100 μL of methanol. The decrease in absorption was measured at 517 nm after 1 h at room temperature. The actual decrease in absorption induced by the test compound was calculated by subtracting that of the control. The concentration required for 50% reduction (SC50) was determined graphically. All the spectrophotometric measurements were performed with a SAFAS UV-mc2 spectrophotometer, equipped with a multicell/multikinetics measuring system and with a thermostated cell-case.
2.6. Statistical Analysis
- Data from three independent replicate trials were subjected to statistical analysis using the Statistica .06, Statistical package [15]. Differences between means were tested using Duncan Multiple Range Test.
3. Results
3.1. Chemical Analysis of Essential Oil
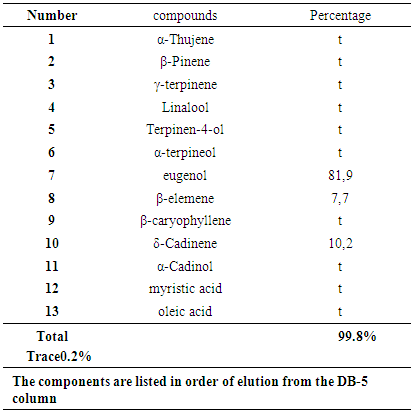
- The essential oil obtained by hydrodistillation of the dry fruits of S. aromaticum presented a yield of 7.6% (v/w). The analysis of this essential oil by gas chromatography made it possible to identify 13 compounds as cited in table 1 in order of elution from the DB-5 column. As shown on the table, the chemical composition of the essential oil of S. aromaticum is dominated by eugenol (81.9%), followed by δ - Cadinene (10.2%) and finally β - elemene (7.7%). These 3 compounds represent approximately 99.8% of the total components of the essential oil of S. aromaticum. The other components of this essential oil are present in traces (<0.2%).
|
|
|
3.2. Antifungal Activities
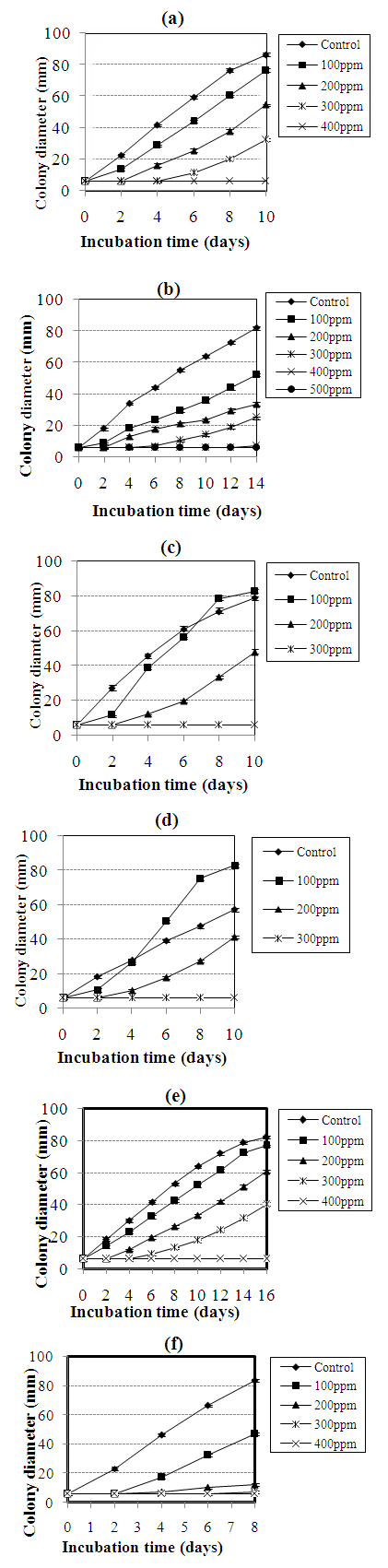
- During the incubation period, average values of daily measurements of the diameter of mycelial growth of the moulds were used to follow-up the growth pattern according to the concentration of essential oil.
3.2.1. Effect of 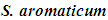 Essential Oil on All Fungal Strains
Essential Oil on All Fungal Strains
- At initial concentrations of 0, 100, 200, 300, 400, 500, 600, 700, 800, 900 and 1000 ppm, the growth diameter of Aspergillus niger, Aspergillus carbonarius, Aspergillus fumigatus, Aspergillus versicolor, Aspergillus flavus and Fusarium oxysporium were recorded and are illustrated on figure 1.
3.2.2. Minimum Inhibitory Concentration (MIC)
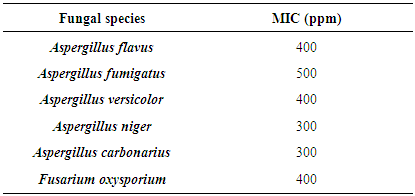
- After noting the concentration at which minimum inhibition was observed from the preliminary tests, the MIC determined for the essential oil of S. aromaticum against all fungal strain are shown on table 2.S. aromaticum essential oil exhibited the lowest MIC values with 300 ppm against Aspergillus niger, Aspergillus carbonarius and 400 ppm against Aspergillus flavus, Aspergillus versicolor, Fusarium oxysporium. S. aromaticum essential oil was less active against Aspergillus fumigatus with MIC value of 500 ppm.
3.3. Antiradical Activities of Essential Oil
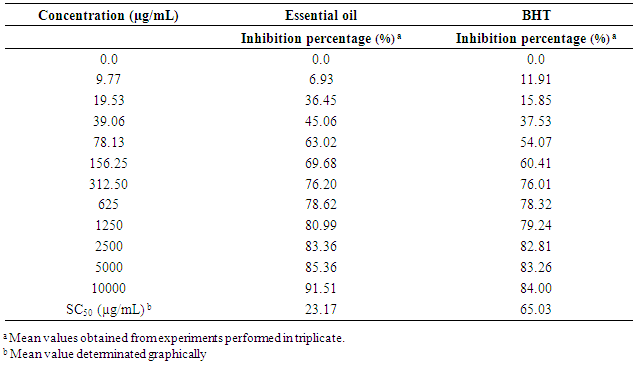
- The results provided by DPPH- test made it possible to obtain the table 3. The concentration which provides 50 % of inhibition (SC50) was used in order to compare the antiradical activity of the essential oil of S. aromaticum with that of the commercial antioxidant molecule (BHT) used as preservative. The results obtained are given in table 3. Generally, it was observed that the scavenging capacity of the essential oil and BHT increases with their concentration in the reaction medium. The following results were obtained: SC50 (BHT) = 65.03 ± 0.99 µg/mL and SC50 (essential oil) = 23.17 ± 0.58 µg/mL. These results indicate that S. aromaticum essential oil is 2.8 times more active than the BHT.
4. Discussion
- In the present study, S. aromaticum essential oil is rich in eugenol (81.9%), δ - Cadinene (10.2%) and β - elemene (7.7%). This chemical composition is different from that collected in other areas. Indeed, Viuda-Martos et al. [10] examined the chemical composition of essential oils of the dry fruits of S. aromaticum collected in the area of Ravetllat Aromatics, (Barcelona, Spain). They found 4 components with a prevalence of eugenol (85.5%), β - caryophyllene (10.54%) and the α - humulene (3.12%). This difference in composition is probably due to conditions such as the environment, the genotype, the geographical origin, the period of harvest, the place of drying, the temperature and the duration of drying, the parasites and the method of extraction [16].Growth of Aspergillus sp. and F. oxysporium in some foodstuffs are considered as health hazards. With increasing consumer demand for naturally preserved food, examination of essential oils for antimicrobial properties has become attractive to researchers and food processors [17]. In vitro results obtained in the present study suggest that essential oil of S. aromaticum might be useful agents for control of Aspergillus sp. and F. oxysporium growth.Results obtained show that S. aromaticum essential oil exhibited the lowest MIC values with 300 ppm against Aspergillus niger, Aspergillus carbonarius and 400 ppm against Aspergillus flavus, Aspergillus versicolor, Fusarium oxysporium. S. aromaticum essential oil was less active against Aspergillus fumigatus with MIC value of 500 ppm.The significant bioactivity obtained in this present study with the essential oil of S. aromaticum could be in relation to its high percentage of eugenol (81.9 %) which is known for its strong antimicrobial activity [18]. Eugenol causes the morphological deformations of the mycelium while acting on the enzymes of the cell wall, such as the chitinases and glucanases [19]. In addition, some components that occur in lesser amount may also contribute to the antifungal activity of the oil, involving probably some type of synergism with the other active compounds.The antifungal activity of essential oil used in the present study is different from those found by other authors who have used the same method. Viuda-martos et al. [20] showed that the essential oil of Syzygium aromaticum of Spain completely inhibits the mycelial growth of A. niger and A. flavus as from 330 ppm. Rana et al. [21] had a MIC of 10000 ppm on Aspergillus sp. and F. oxysporum with the essential oil of S. aromaticum collected in India. This difference in activity could be explained by the chemical profile of these essential oils, but also by possible synergistic interactions or antagonistic interactions between the various components present in each essential oil at the origin of an activity much more significant or weaker [22].Results obtained also show that, the antifungal activity of S. aromaticum essential oil is not general for all fungal strains, for A. niger and A. carbonarius, the growth of the control was less than that of the sample with 100 ppm of S. aromaticum essential oil. This could be related to the presence of nutritive substances in essential oils such as fatty acids [23, 24]. A. niger and A. carbonarius produce the lipases which break down fatty acids, which could explain a faster growth compared to the control.It was also observed that MICs of S. aromaticum essential oil against all fungal strains varied with incubation time. For example, it ranged from 200 ppm, after 2 days of incubation, to 300 ppm after 10 days for A. niger and 400 ppm for A. flavus respectively. This could be due to the fact that during a relatively long incubation period some volatile components in these oils may evaporate from the media, leading to decrease in their concentration [25].Interest has increased considerably in finding naturally occurring antioxidant for use in foods to replace synthetic antioxidants, which are being restricted due to their side effects such as carcinogenicity [26]. Several authors found that the natural antioxidants can protect the human body from free radicals and retard the progress of many chronic diseases as well as retard lipid oxidative rancidity in foods [27]. The oil of S. aromaticum showed a greater radical scavenging capacity (SC50) than the BHT. This greater SC50 of S. aromaticum essential oil could be explained by its chemical profile; mainly rich in eugenol (81.9%), but also by synergistic interactions between eugenol and the other minority components present in this essential oil [28]. The antiradical effectiveness of eugenol can be explained by the fact that it acts by three main mechanisms of action [14]: donation of hydrogen followed by the delocalization of the group substituted at the para position; dimerization between two phenoxylated radicals and complexation of DPPH˙ with an aryl radical.The results of this present study are in agreement with those of Muhammad et al. [11] which showed that the antiradical potential of S. aromaticum essential oil (SC50 = 4.56 ± 1.07 µg/mL) which originated from Pakistan was more effective than that of BHT (SC50 = 2.1 ± 0.92 µg/mL), but these results are contrary to those obtained by Khunkitti et al. [29].
5. Conclusions
- The results of this study show a great antifungal activity of the essential oil of S. aromaticum on the mycelial growth of A. flavus, A. fumigatus, A. niger, A. carbonarius, A. versicolor and F oxysporium, with minimum inhibitory concentration (MIC) about 300 to 500 ppm. Among all these moulds, A. fumigatus is the most resistant. The evaluation of the antiradical activity of the essential oil of the dry fruits of S. aromaticum in comparison with a reference antioxidant (BHT) showed that this essential oil has an antiradical activity of 2.8 times more active than that of BHT. These results show a possibility in exploiting the essential oil of S. aromaticum as an alternative solution to synthetic fungicides and antiradicals used for the conservation of agro-food products.
ACKNOWLEDGEMENTS
- Authors are grateful to University of Ngaoundere (Cameroon) for support in the form of infrastructural facilities made available for undertaking the present study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML