-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(6): 169-174
doi:10.5923/j.microbiology.20150506.01

Cellulase and Biomass Production from Sorghum (Sorghum guineense) Waste by Trichoderma longibrachiatum and Aspergillus terreus
Olaleye Oluremi Nurudeen1, 2, Omotayo Mutiat Adetayo1, Abdus SalaamRofiat Bolanle3, Olanlege Abdul-Lateef Olaltunde1
1Department of Science Laboratory Technology, Lagos State Polytechnic, Ikorodu, Lagos, Nigeria
2SubSaharan Centre for Environment & Water Research Innovations and Development (SCEWRID)
3Department of Food Technology, Lagos State Polytechnic, Ikorodu, Lagos, Nigeria
Correspondence to: Olaleye Oluremi Nurudeen, Department of Science Laboratory Technology, Lagos State Polytechnic, Ikorodu, Lagos, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The production of single cell protein and cellulase from cellulosic sorghum waste using Trichoderma longibrachiatum and Aspergillus terreus was assessed. Pre-treated waste was added to basal media, inoculated with test organisms and incubated at 30°C in a New Brunswick G24 gyratory shaker at 180rpm. Samples were withdrawn at intervals of 24hours for 120hours and were subjected to protein assay and mycelia weight determination. The activity of cellulose produced by each organism was determined using filter paper and carboxymethyl cellulose as substrate. Optimum pH and temperature were also determined to validate the reaction conditions of the enzymes. Trichoderma longibrachiatum and Aspergillus terreus grew well on the sorghum waste producing a mycelia weight of 0.74g and 0.81g with protein concentration of 1.63mg/ml and 0.71mg/ml at 120hours respectively. Cellulase produced by test organisms degraded filter paper and carboxymethyl cellulose with Trichoderma longibrachiatum producing an activity of 1.46U/mL and 1.82 U/mL respectively while Aspergillus terreus cellulase produced activity of 1.46U/mL and 0.95U/mL respectively. However, optimum pH for cellulase activity Trichoderma longibrachiatum and Aspergillus terreus were 5.0 and 4.0 respectively while optimal temperature for cellulase activity from the two organisms was 50°C.
Keywords: Cellulolytic enzyme, Biomass, Aspergillus terrus, Trichoderma longibrachyatum
Cite this paper: Olaleye Oluremi Nurudeen, Omotayo Mutiat Adetayo, Abdus SalaamRofiat Bolanle, Olanlege Abdul-Lateef Olaltunde, Cellulase and Biomass Production from Sorghum (Sorghum guineense) Waste by Trichoderma longibrachiatum and Aspergillus terreus, Journal of Microbiology Research, Vol. 5 No. 6, 2015, pp. 169-174. doi: 10.5923/j.microbiology.20150506.01.
1. Introduction
- The development of a community, society or nation characterized by rapid urbanization and industrialization comes with the deleterious effect of environmental pollution [1] and loss of large expanse of land available for agricultural use. Agro wastes are biodegradable and renewable waste material produced in large quantities from agricultural practices and agro-allied based industries such as textile, paper and pulp, breweries industries [2]. Fermentation of grains in brewing industries and other food processing industry generates solid waste in form of spent grains called bagasse [3] which was estimated to contain 70% fibre in the form cellulose, hemicellulose, arabinoxylans and lignins [4, 5]. Cultivation of microorganism on agro waste has been reported to be a promising approach for increasing the availability of proteins via single cell protein production [6, 7, 8] to meet the requirements of burgeoning populations in Nigeria and several areas of the world; reducing pollution and producing enzymes of biological and industrial importance due to their affordability and abundance in nature [9].Cellulases are important carbohydrate hydrolytic enzyme with great importance in biotechnology and vast usage in the textile, pulp and food industries [10, 11, 12]. The cellulase enzyme complex hydrolyses cellulose and hemicellulose, the polysaccharides found abundantly in agro waste to its monomeric units, glucose which can be utilized in the production of various useful materials including ethanol and biofuel. It has been successfully synthesized by various microorganisms on different forms of agro wastes including sugarcane bagasse [13], guinea corn stalk, millet straw, rice husk and saw dust [14], and corn cob [15]. With the abundance of wastes generated in Nigeria from processing of sorghum to ogi, this work evaluates the possibility of producing single cell proteins using sorghum waste as substrate and the concomitant synthesis of the enzyme, cellulase.
2. Materials and Methods
- Sample collection Large quantity of sorghum waste (from ‘Ogi’) was obtained from three different localities (Ketu, Mushin and Ebute-metta) in Lagos metropolis of Nigeria [14].Micro-organism sourceAspergillus terreus and Trichoderma longibrachiatum were obtained from the culture collection unit of the Biotechnology Division, Federal Institute of Industrial Research, Oshodi. The organisms were maintained on potato dextrose agar slant at 4°C and sub cultured on the PDA plates.Culture conditionTreatment of sorghum extract 20g of sorghum wastes in 100ml of 10% sodium hydroxide (NaOH) was pretreated in an autoclave at 121oC for 30minutes. The extract was neutralized with H2SO4 [16]. Preparation of the basal medium: The salt solution containing (g/l) yeast extract 10g; (NH4)2 SO4 0.9g; KH2PO4 2.0g; K2HPO4 1.0g; Fe SO4 0.16g; MgSO4.7H2O 0.2g; MnSO4 0.12g and sorghum extract 20g [16]. 10g each of pre-treated and neutralized sorghum were added into salt medium. The basal medium was sterilized at 121°C for 15 minutes. Potato dextrose agar was prepared and autoclaved at 121°C for 15minutes after which streptomycin 0.014g/L was added to the medium.Inoculation The prepared culture containing 50ml salt medium plus 1% glucose as the carbon source were inoculated with mycelia of 4 day old cultures of Aspergillus terrus and Trichoderma longibrachiatum were incubated on a shaker for 18 hours at 30°C. 1ml sample of the actively growing starter culture were used to inoculate media shake flask and were incubated at 30°C in a New Brunswick G24 gyratory shaker at 180rpm. Samples were withdrawn at intervals of 24hours for 120hours (5 days) for assay. Harvesting myceliumEach biomass produced was harvested by vacuum filtration of the culture brought through a 25mm pore size nylon cloth. Followed by centrifugation at 5000rpm for 20minutes.The dry weight of biomass was determined by drying the washed residue from the vacuum filtrate of broth at 103°C for 6hours.Protein assay30mg dried grounded mycelia were hydrolyzed with 2.5ml of 6N HCl in tubes for 20hours at110oC. Hydrolysates were cooled at room temperature, neutralized with 6N NaOH, diluted to 50ml with distilled water and filtered through whatman No. 2 filter paper. Protein was estimated after reacting 0.1ml aliquots of protein extract with 4.0ml of a solution consisting of 1 volume of trinitrobenzene sulfonic acid (1.0g/L) and 3 volume of buffer (21.0g NaHCO3 + 26.5g Na2CO3/L). The tubes were incubated in the dark at a room temperature for 60minutes and absorbance was determined with a spectronic 20D spectrophotometer set at a 390nm wavelength. Protein was extrapolated for a standard curve constructed using bovine serum albumin (BSA) (sigma chemical) [17].Determination of cellulase activityCulture supernatants, obtained by centrifugation at 5000rpm for 20minutes, comprised the crude enzymes preparations. Carboxymethylcellulose and filter paper activity were determined according to Mandels and Weber [18].Determination of cellulase activity• Carboxymethylcellulose:The assay consisted of 10mg of carboxymethylcellulose sodium salts dissolved in 1.6ml of 0.1M citrate-phosphate buffer (pH 6.0) culture supernatant (0.2ml) was added to pre warmed substrate and tubes were incubated at 45oC for 30minutes. Adding 4ml of DNS reagent stopped the enzyme action. The resulting solution was boiled in a water bath for 5minutes. The volume of the solution was made up to 10ml with distilled water and cooled under running tap water. The amount of reducing sugar formed was determined by taking the optical density of the solution at 540nm against a blank of distilled water treated as above. The reducing sugar content was calculated from a standard curve of glucose treated with DNS reagent.• Filter paper Activity: The assay consists of whatman No. 1 filter paper strips (6 by 1cm to give 50mg) coiled in the bottom of the test tube to which 1ml of 0.2M Sigma-Aldrich citrate-phosphate buffer (pH 6.0) was added. Culture supernatant (1.0ml) was added to pre warmed substrate and the reaction mixture was incubated at 45°C for 4 hours. The reaction was terminated by addition of 4ml of DNS reagent. The reaction mixture was boiled in a water bath for 5minutes. The volume of solution was made up to 10ml with distilled water. The amount of the reducing sugar formed was determined by taking the optical density of the solution at 540nm against blank solution of distilled water. The reducing sugar content was calculated from a standard curve of glucose.Effect of pH on cellulase activity The enzymes hydrolyzed CMC in the range of pH 3 to pH 7. This was monitored in buffer solution of various pH values and the reducing sugar formed was estimated with DNS reagent.Effect of temperature on cellulase activityThe enzyme was assayed at different temperatures in universal buffer (pH 6.0) at Temperature of 25°C to 60°C. The enzyme was measured by heating 100ml of the enzyme solution (30g) in 0.05M citrate-phosphate buffer for 30 minutes at the indicated temperature range and the reducing sugar formed was estimated with DNS reagent [19].
3. Result and Discussion
- Single cell protein are dried cell mass of fungi, moulds and bacteria used as protein supplement in animal and human feed to augment their diet [20]. A. terreus employed in this study have been used by other researchers to produce single cell proteins [21, 22] on different substrates. Maximum mycelia weight was achieved at 96hours with A. terreus giving a higher yield in comparison to T. longibrachiatum. However, from protein concentration assay of the dried mycelia weight shown in figure 2, T. longibrachiatum had higher protein content than A. terreus. Cellulase are produced by different species of bacteria and fungi [23], however, fungi are more explored [24]. A. terreus and T. longibrachiatum utilized in this study are proficient cellulase producer as substantiated by the production of glucose shown in figure 2. Both organisms showed gradual progression in glucose concentration due to increase in rate of enzyme secretion and substrate hydrolysis, but, optimal glucose concentration was observed at 120 hours for both organisms. However, rate of hydrolysis is expected to fall due to exhaustion of cellulose, enzyme inactivation and product inhibition.
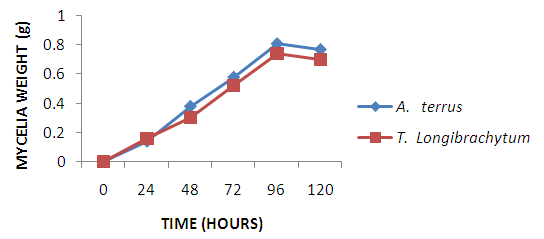 | Figure 1. Weight of dried mycelia |
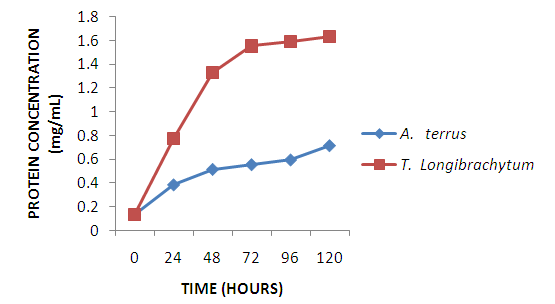 | Figure 2. Protein concentration of dry mycelia |
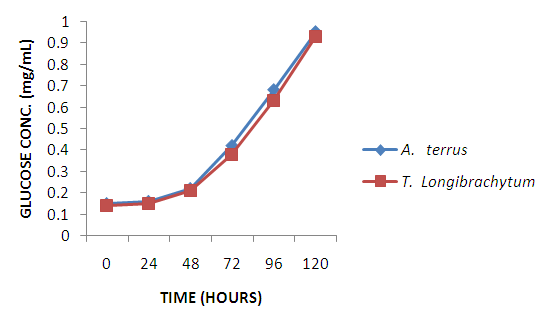 | Figure 3. Fermentation of sorghum wastes by test organisms |
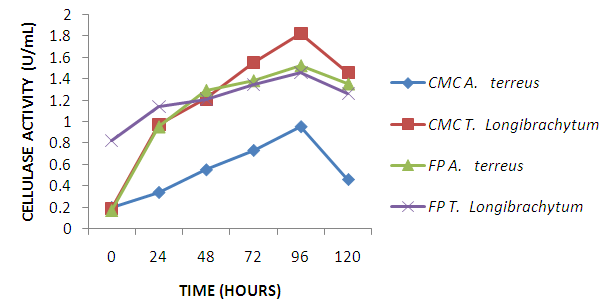 | Figure 4. T. longibrachiatum and A. terreus cellulase activity on CMC and FP |
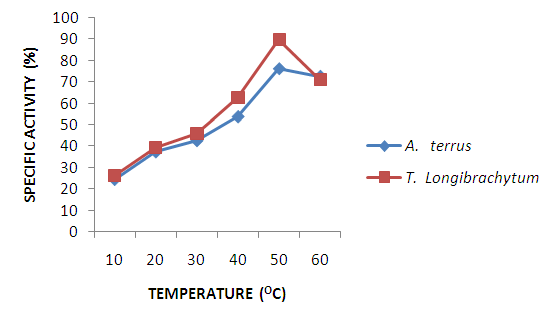 | Figure 5. Effect of temperature on specific activity of cellulase using CMC as substrate |
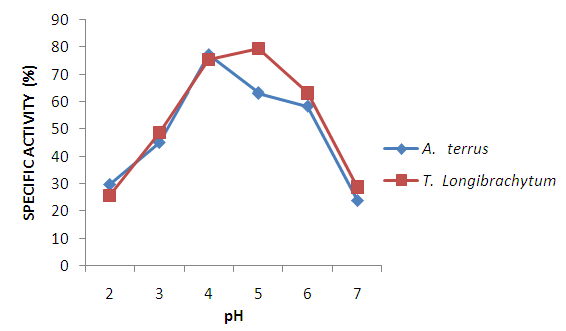 | Figure 6. Effect of pH on specific activity of cellulase using CMC as substrate |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML