-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(5): 161-168
doi:10.5923/j.microbiology.20150505.04
Composition of Antibiotic Resistant Bacteria from Irrigated Vegetable Farmland
Oluyege J. O., Oluwaniyi T. T., Ijasan O. C.
Department of Microbiology, Faculty of Science, Ekiti State University, Ado-Ekiti, Nigeria
Correspondence to: Oluwaniyi T. T., Department of Microbiology, Faculty of Science, Ekiti State University, Ado-Ekiti, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The study aim to investigate the microbial quality and incidence of antimicrobial resistance of bacteria isolated from vegetable farmland in Ekiti State University, Ado-Ekiti (7° 42’41’’N 5°14’59” E).The farm soil (300square metre) was treated with poultry droppings at 500g/square metre before it was cultivated. Five species of vegetables including black and white Amaranthus spp, Albemoschus esculentus (okro), Lycopersicum esculentum (tomato) and Telfairia occidentalis (ugwu) were cultivated on the treated soil. Wastewater effluent from fish pond near the farmland served as source of irrigation. The soil, wastewater, poultry droppings and the vegetables harvested were analysed. Total bacteria count (TBC) of the samples ranged from 8.0x105CFU/ml to 1.2x105CFU/ml, and the mean was 5.6x105CFU/ml. The total coliform counts ranged from 4.1x102CFU/ml to 1.0x103 CFU/ml and the mean was 2.6x103CFU/ml. Eighty-eight bacteria of seven genera comprising Bacillus spp, (33%), E. coli (18.2%), Staphylococcus aureus and Pseudomonas spp (14.77%), Klebsiella spp(12.5%), Proteus spp(4.5%) and Corynebacterium(2.27%) were isolated. All the bacteria isolated exhibited varying patterns of resistance against the screened antibiotics namely: gentamycin (10%), ciprofloxacin (20%), ceftazidime (48%), cefotaxime (50%), perfloxacin (29%), cefuroxime (53%), ceftriaxone (43%), erythromycin (71%), vancomycin (44%) and penicillin (55%). Hence, the consumption of vegetables harvested from the study site is a threat to public health because they are carriers of antibiotic resistant bacteria that may cause infections difficult to treat.
Keywords: Ado-Ekiti, Antibiotic Resistance Bacteria, Farmland, Poultry Droppings, Soil, Vegetables
Cite this paper: Oluyege J. O., Oluwaniyi T. T., Ijasan O. C., Composition of Antibiotic Resistant Bacteria from Irrigated Vegetable Farmland, Journal of Microbiology Research, Vol. 5 No. 5, 2015, pp. 161-168. doi: 10.5923/j.microbiology.20150505.04.
Article Outline
1. Introduction
- Wastewater includes all water in which the quality has been adversely affected by the influence of human activities (Burston and Stensey, 2003). It includes wastewater discharged from domestic residences, commercial, hospital, industrial or agriculture processes (IWMI, 2010). Moreso, it includes water containing human excretes and urine, known as ‘black water’ from indiscriminate disposal in bushes and farmlands, septic tanks and washing water; while greywater contain runoffs from roads, roofs and sidewalks (Simon-Oke et al., 2014; Abdullahi et al., 2013). Excretes from free grazing animals may be washed as runoffs into surface water or when applied as manure on crop-cultivated lands. Wastewater may contain different components such as pathogens, synthetic chemicals, organic matter, nutrients, organic compounds and heavy metals; occurring either in solutions or as particulate matter. Availability of good quality water for irrigation is limited worldwide; hence agricultural practice is constrained to make use of municipal wastewater (Palese et al., 2009). Thus, water supply for agriculture is increased. Besides, the reuse of wastewater is an option for water conservation. Consequently, farmers involved in urban agriculture in developing countries have adopted the use of wastewater for irrigation; and this is becoming a popular practice in these countries (Sou et al., 2011). Wastewater provides water, significant amount of organic matter and micronutrients for plants use; promoting crop/plant growth and yield (Noori et al., 2014; Singh et al., 2011). Therefore it encourages water conservation, nutrients recycling, reduction in the application of inorganic fertilizers to soils and reduction in discharged of polluted water into water bodies (Vasudevan et al., 2010). The use of untreated and partially treated wastewater for irrigation is intense worldwide especially in arid, semi-arid regions and urban areas where unpolluted water is scarce and wastewater enriched with nutrients serve as an important drought-resistant resource for farmers (WHO, 2006; Ackerson and Awuah, 2012; Scott et al., 2004). Research has estimated that twenty million hectares in 50 countries of the world are irrigated with raw or partially treated wastewater, and 200 million farmers are involved. The estimate is likely to increase markedly in the next few decades as pressure on water intensifies (Hussein et al., 2001; Hamilton et al., 2007; Raschid-Sally and Jayakody, 2008). However, though wastewater agriculture importantly contributes to urban food supplies and helps provide a livelihood for the poor, it may introduce diverse elements and inorganic minerals that are potentially toxic to human and animal health; and may cause crop quality deterioration (Zavadil, 2009). It may also contain heavy metals posing health risk to animals and human when ingested in sufficient concentrations. In addition, untreated wastewater is highly contaminated with pathogenic bacteria, coliforms, intestinal helminthes and their eggs (Hussain et al., 2002); and are responsible for chronic diseases that have long term effects such as degenerative heart diseases and stomach ulcer (Paillard et al., 2005). More so based on its numerous point sources, wastewater streams comprises antibiotic resistance bacteria that have been given perfect opportunity to swap and transfer resistant genes over the time. These bacteria find their ways to the agricultural soils when such water is channelled to the land for reuse. More so, the exposure of soil bacteria to antimicrobial drugs applied during agricultural practices may enhance their antibiotic resistance pattern. The abundance and mobility of antibiotic resistance genes in agricultural soils may also be enhanced by various management practices such as, the application of animal manures and wastewater containing antibiotic residues and antibiotic resistance genes on mobile elements (Jechalke et al., 2013; Gaze et al., 2011; Heuer et al., 2011; Wellington et al., 2013). These elements include plasmids, integron and transposons. Since antibiotics are used in livestock and crop production as growth promoter and as therapy against infections in animal husbandry, there is engendered concern that the application of animal manure on agricultural lands cultivated for crop production can potentiates and disseminates antibiotic resistance bacteria to crops intended for animal or human consumption about (Siwela et al., 2007). Vegetables such as corn, green onion and cabbage, absorb antibiotics when cultivated in soil fertilized with antibiotics contaminated manure from livestock (Kumar et al., 2005). Hence, the application of wastewater in crop irrigation poses food and health safety problem to human and animal lives (CDC, 2005); particularly when farm produce from these farmlands such as vegetables are consumed raw or uncooked. Antibiotic resistance is rising in some bacterial pathogens such as Pseudomonas, Corynebacterium and Klebsiella, the causative agents of some foodborne illness. Antibiotics such as fluoroquinone, methicillin and cephalosporin are becoming increasing less effective against the infections caused by these pathogens (CDC, 2005). Recently some antimicrobial-resistant pathogens have emerged in the food-production chain: extended beta-lactamase producing Escherichia coli, and animal-associated methicillin-resistant Staphylococcus aureus (MRSA), which can transmit and cause infections in humans (Aarestrup et al., 2008; Xia et al., 2010). Secondly, more number of healthy people become ill when infected with few numbers of pathogens that are usually not responsible for the illness. Ordinarily, healthy persons who consume few Salmonella may not show any symptoms, because the indigenous bacteria in their intestines keep the pathogens in check. However, consumption of few antibiotic-resistant Salmonella in foods can cause illness if consumers take an antibiotic for another reason (CDC, 2005). Antibiotic resistance infections require prolonged and costlier treatments and may result in death or disability when compared with infections caused by ordinary bacteria that have not taken up the genes (CDC, 2005). It has been reported from several researches that majority of the antibiotic resistant bacteria originate from hospitals and eventually reach the municipal sewage wastewater. Crops become contaminated by these resistant bacteria when the water is reused as irrigant or for washing. Subsequently, they may spread through an entire population endangering their health. Although, the use of wastewater serving as alternative water source and poultry droppings serving as manure are cheap options in farm management, there is need to understand associated microbial risk arising from the incidence of antibiotic resistant bacteria from such land. This study therefore seeks to determine the antibiotic resistant bacteria present in the poultry droppings, irrigation water, soil and the farm produce.
2. Materials and Methods
2.1. Preparation of Farmland for Study
- The study area is a vegetable farm located within Ekiti State University, Ado-Ekiti, Ekiti State, Nigeria (7°42’41’’N 5°14’59” E). Untreated fish pond wastewater from the University campus drains into receiving-stream near the vegetable farm. Faeces from grazing cattle around the farm regions and indiscriminate excretes from human were observed in the farm surrounding. In preparation to cultivation, poultry droppings (organic manure) collected from the University poultry farm was mixed with the farmland soil (300sq.metre) at 500g/sq.metre. Seeds of five different species of vegetables were sowed on the tilled land. They include white and black Amaranthus spp, Albemoschus esculentus (okro), Lycopersicum esculentum(tomato) and Trifela occidentalis (ugwu). The cultivation was done between September and November, 2014 during which the dry season was at the peak at atmospheric temperature of 30°C± 2°C. The farmland was irrigated with untreated fish pond wastewater receiving stream. The vegetables were harvested after maturity and microbiological analyzed along with the other environmental samples from farmland.
2.2. Collection of Samples
- A total number of 8 samples including five vegetables, wastewater, soil and poultry manure were collected from the farmland. Samples were aseptically collected in sterile universal containers maintained at 4°C in a cooler box and taken to the laboratory of Department of Microbiology, Faculty of Science, Ekiti State University for microbiological examination.
2.3. Preparation of Samples for Microbiological Analyses
- Ten grammes each of the bulk solid samples; vegetables, soil and poultry droppings) were homogenised in sterile peptone water (90 ml). The homogenate served as the primary dilution. Ten-fold dilution of the homogenate of each of the samples was done using physiological saline. Dilution 10-5 (1.0ml) was mixed with molten standard plate count agar, Eosin Methylene blue or MacConkey agar at 45°C and then poured into sterile Petri-dish plates. The plates were allowed to set and incubated at 37°C for 18h. Wastewater (1ml) was sterile-pipetted in sterile peptone water (9 ml) in sterile test tube; it was carefully swirled to obtain uniform mixture, and then a series of dilution was done till 10-5 dilution factor. The inoculation and incubation of the wastewater diluent was done as described for solid samples above. The developed colonies of bacterial species on the media used were counted and recorded.
2.4. Identification of Bacterial Isolates
- Positive cultures were subcultured severally on fresh plates of sterile selective agar for pure single colonies. The isolates were identified using standard microbiological methods. The biochemical tests were carried out using standard bacteriological techniques (Barrow and Feltham, 2003). Biochemical tests conducted were Oxidase Test, Catalase Test, Coagulase Test, Indole Test, Urease Test, Carbohydrate fermentation/Utilization test, Hydrogen Sulfide production (H2S), Methyl Red (MR) test, Voges-Proscauer (VP) test, Citrate Utilization test, Nitrate reduction test, Indole production and Urea (MIU) tests.
2.5. Antibiotic Susceptibility Test
- Commercial disks (Oxoid, UK) used in this study contain eleven antibiotics: Cefotaxine 30 µg, Ceftazidime 30µg, Ciprofloxacin 5µg, Chloramphenicol 30µg, Gentamicin 10µg, Penicillin G 30µg, Vancomycin 30µg, Erythromycin 5µg, Perfloxacin 10µg, Ciprofloxacin 30µg and Cefuroxine 30µg. The antibiotic susceptibility of the bacterial isolates was investigated by the disk diffusion method and according to the standard microbiological guidelines CLSI (2007). Isolates were subcultured on freshly prepared nutrient agar, and incubated at 37°C for 24 hr. 3-5 distinct colonies of each organism were picked with a sterile wire loop and emulsified in a 5ml of sterile physiological saline; the turbidity of the suspension was compared with McFarland Turbidity Standard of 0.5. Petri dishes with sterile Mueller-Hinton agar (4 mm depth) were used for the test. Streaking of the bacterial suspension with a clinical swab was done on the entire agar. The bacterial suspensions were aseptically streaked uniformly on the entire agar surface using sterile swab in three different directions by rotating the plate at 60° angles after each streaking. The petri plates were left to dry for 20 min. Afterwards, the standard commercial paper discs of selected antibiotics were placed on the agar surface with a sterile forceps and pressed carefully down to ensure contact. The plates were inverted and incubated aerobically at 35°C for 16-18hours. The plates were examined after incubation for diameters of zone of inhibition around the discs, which were measured using micrometre screw-gauge. The data obtained were interpreted with reference to CLSI (2007).
3. Results
- All the samples including the fresh vegetables sampled recorded high levels of total bacterial and coliform count presented in Table 1. The total bacteria count (TBC) of the samples ranged from 8.0x105CFU/ml, in poultry droppings to 1.2x105CFU/ml, in Telfairia occidentalis (ugwu), with mean 5.6x105CFU/ml. Meanwhile, the total coliform counts (TCC) ranged from 4.1x102 CFU/ml, in soil to 1.0x103 CFU/ml, in ugwu with the mean of 2.6x103 CFU/ml. The TBC and TCC were noted to be least in ugwu leaves but highest in poultry droppings and soil respectively. A total number of 88 bacteria that are members of seven genera were isolated from the samples. The frequency of occurrence of bacteria is presented in Table 2. Bacillus spp (33%) occurred most frequently, followed by Escherichia coli (18%), then both Pseudomonas spp and Staphylococcus aureus (15%), Klebsiella spp (13%), Proteus spp (4%) and the least frequently isolated was Corynebacterium spp (2%). The percentage distribution of bacteria isolates in all the samples are presented in table 3. The overall antibiotic resistant pattern of the bacterial isolates to the following antibiotics is represented in table 4: Ciprofloxacin, Ceftazidime, Cefotaxime, Perfloxacin, Cefuroxime, Ceftriaxone, Erythromycin, Vancomycin, Penicillin and Gentamycin. Figure 1 presents total isolates from each sample; they all demonstrated multiple resistances to antibiotics. They comprises 17 in wastewater; 9 in tomatoes; 14 in poultry droppings; 10 in black vegetable; 8 in white vegetable; 8 in okro; 14 in soil and 8 in ugwu. Figure 2 represents the overall percentage of bacterial isolates and total antibiotics they are resistance to.
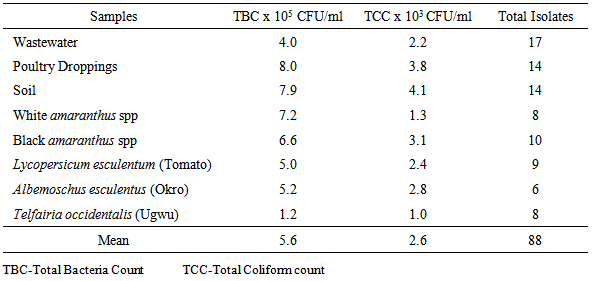 | Table 1. Total plates counts of samples from wastewater-irrigated and manure-treated vegetable farmland |
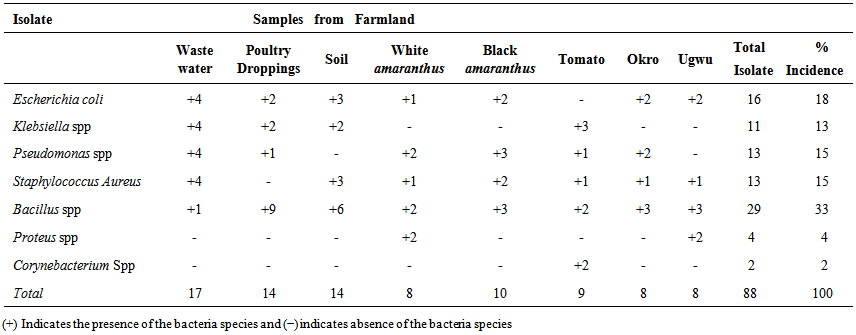 | Table 2. Occurence of bacteria isolates from wastewater-irrigated and manure-treated farmland |
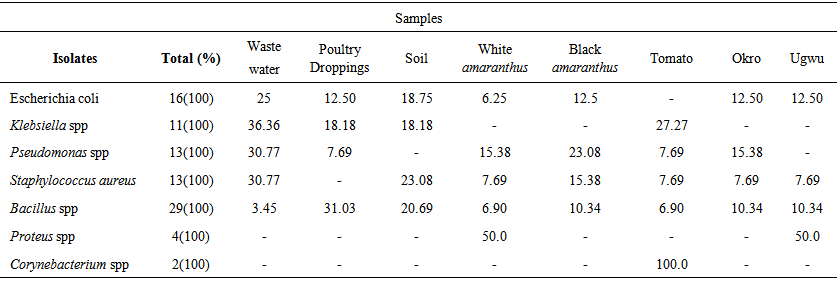 | Table 3. Percentage distribution of bacteria isolates in samples from wastewater-irrigated and manure-treated farmland |
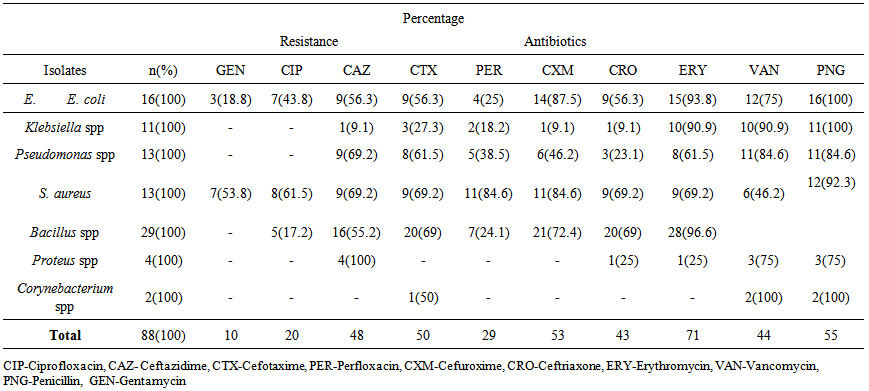 | Table 4. Antibiotics resistance pattern of bacterial isolates from samples from the vegetable farmland |
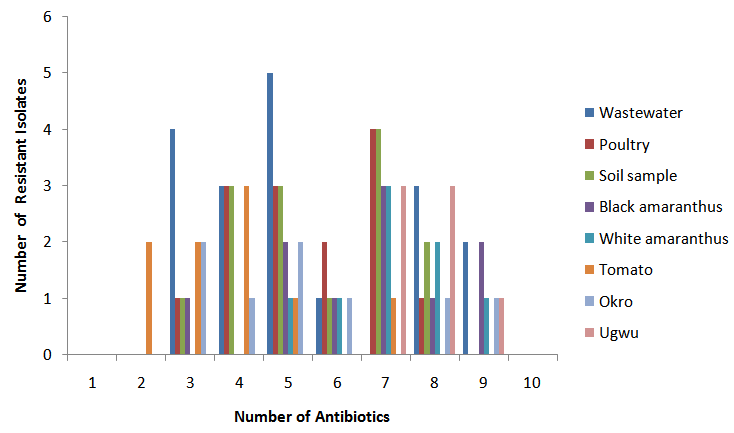 | Figure 1. Overall multiple antibiotic resistance isolates in samples |
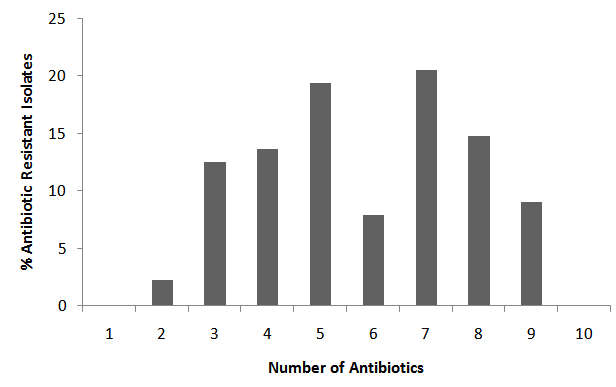 | Figure 2. Overall Percentage Multiple Antibiotic Resistant Isolates |
4. Discussion
- The high incidence of bacteria in all the samples is not surprising as bacteria are ubiquitous and are expected in all unsterile environments. Bacillus spp occurred in all the samples; E.coli and Staphylococcus aureus were isolated from all the samples except tomatoes and poultry faeces respectively. Pseudomonas spp were detected in all samples except soil and ugwu; Klebsiella was present in wastewater, poultry faeces and tomatoes. Corynebacterium spp was not detected in any of the samples except tomatoes; while Proteus spp occurred in the white amaranthus and ugwu only. E. coli was highest in wastewater (20.69%), Bacillus spp (31.03%) in poultry droppings and Klebsiella spp (36.36%) in wastewater. Both Pseudomonas spp and Staphylococcus aureus (30.77%) were highest in wastewater, while Corynebacterium ssp was 100% in tomato. The untreated wastewater contained numerous bacterial pathogens; and being directly used as source of water and minerals in vegetable-cultivated farmlands would definitely contribute to the high bacterial contents of the soil and the farm produce. The higher bacteria counts recorded on the white and black amaranthus in comparison with tomatoes, okro and ugwu, is as a result of their large surface areas. The high total coliform counts observed in all the samples indicate the presence of fecal bacteria and other intestinal bacteria attributed to untreated wastewater effluents. A high coliform count in poultry droppings is expected because almost all the bacteria are of fecal origin. Also, the indiscriminate faeces in surrounding bushes washed off by rain to farm soils must have contributed to the high coliform counts observed the soil and wastewater. The isolation of coliforms from vegetables was not unexpected, since water used for irrigation is reported to contain coliforms and enteric bacteria (Gagliard and Karns, 2000). The application of fresh poultry droppings also contributed to the high population of coliforms in soil and vegetables. This finding is similar to the the report of Drechesel et al. (2000) and Benti et al. (2014). The wastewater is also responsible for the high coliforms level recorded in the harvested vegetables. Though, the permitted fecal coliforms and non fecal coliform on vegetables are yet to be established; however according to the (ICMSF, 1998) the vegetable analyses showed undesirable level of 1x103 CFU/ml but acceptable residual level of 1x 105. The coliform levels of vegetables correspond to the total coliform in wastewater used for the irrigation. Hence, consumption of these vegetables can result in food infections. The incidence of Bacillus species as the highest occurring bacteria is not surprising; they are saprophytes and possess endospores that enable them to survive harsh physical and chemical environmental conditions. The presence of Escherichia coli on the other hand are indicators of recent fecal contaminants (Nawas, 2012). Pseudomonas aeruginosa is majorly found in water, soil and plant surfaces; it tolerates various physical conditions. It is an opportunistic pathogen, causing several diseases including urinary tract infection and gastrointestinal tract infections (Shigeki Fujitani et al., 2014). The presence of Pseudomonas aeruginosa and Klebsiella pneumonia in the soil and wastewater in this study was not surprising; previous study by Tumeo et al. 2008) and Prado et al., (2008) gave the same report. The contamination of vegetables arises majorly from wastewater used for watering and poultry droppings applied on the farmland. The utilization of untreated wastewater for irrigation of crops on farmlands is associated with a number of risks such as reduction in crop yield, decline in crop quality, crop contamination with pathogens and deterioration of soil properties (Noori et al., 2014). Thus, the isolates fulfilled the criteria to be selected as multiple antibiotic resistant bacteria.A lot of attention has been focused on the roles played by animal-derived foods in the acquisition and persistence of antibiotic resistance in humans (Threlfal et al., 2000), and it is presumed that acquiring antimicrobial resistance from these foods will adversely affect the effectiveness of antimicrobial chemotherapy. In this study, multiple drug resistance was observed in 100% isolates with a resistance to two to nine antibiotics; similar observation was reported by Nipa et al. (2011) and Marti et al. (2013). A prominent etiology of diseases is Staphylococcus aureus, and many of its isolates have become resistant to both synthetic and traditional antimicrobial chemotherapy (Daum and Seal, 2001; Kaplan et al., 2005). The presence of multiple antibiotic resistant Staphylococcus aureus in the vegetables and other samples in this study is therefore a disturbing threat as it may cause food poisoning in consumers and worsen illness in persons with already existing superficial infections (Adegoke and Komolafe, 2009). Murugan et al., (2011) reported that problems are encountered in the treatment of gastrointestinal diseases associated with food and water contaminated by E. coli. This problem may be compounded by its emergence of antibiotic resistance to increasing number of antibiotics (Goettscha et al., 2000). Multidrug resistant Pseudomonas aeruginosa infections have been reported to be associated with severe adverse clinical outcomes (Aloush et al., 2006) that are difficult to treat. Also, majority of Bacillus spp have no pathogenic potential and have not been associated with diseases in man or animals except for few species such as Bacillus cereus that are responsible for food poisoning (Carmelita, 2014), yet the presence of multiple resistance isolates may be a predisposing factor to food borne infection. However, it has been reported by Weber et al., (1988) that some strains of Bacillus species are resistant to penicillins, oxacillin, clindamycin, cefazolin, cefotaxime and cephalosporins.Multiple antibiotic resistance bacteria originate from environments where several antibiotics are in use. The use of antibiotics in animal husbandry and poultry has promoted the development of antibiotic resistance in bacteria in farm environments (Heuer et al., 2011). These resistance genes located in bacteria genome may shift to mobile genetic elements such as integrons, plasmids and transposable elements; and they are horizontally transferred to bacteria adapted to soil or other habitats that may support their environmental transmission (Heuer et al, 2011). Organic manure has been identified as a reservoir of resistant bacteria and antibiotic compounds; and its application to agricultural soils will significantly cause a spread of antibiotic resistance genes to bacterial population in soil and vegetable produce. The prevalence of drug resistant organisms in contaminated vegetables and its consumption contributes to dissemination of multiple antimicrobial resistant bacteria posing a great challenge to clinicians as they may serve to prolong the treatment of food borne diseases.
5. Conclusions
- The result from this study revealed that the poultry droppings and wastewater which served as sources of organic manure and water respectively, might pose great health concern to human; they comprise unacceptable pathogenic bacteria beyond the limit by ICMSF guideline. More so, these bacteria showed resistance to multiple antibiotics posing additional health threat to people especially the immunocompromised. The application of the organic manure and wastewater serves as source of transmission of the pathogenic bacteria to both the soil and vegetables. The result is also significant to cultivation of vegetables, especially when some of them are consumed raw or partially cooked. It is therefore recommended that wastewater should be treated before being dispersed to flowing streams that serve as source of water in farms. Poultry droppings must be allowed to compost to reduce the effect of pathogenicity of the bacteria. Farmers especially those with health challenge should be cautious when handling wastewater during irrigation to avoid being infected. It is advisable to properly wash vegetables and other farm products before consumption.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML