Aluoch AM1, 2, Obonyo MA2, Okun DO3, Akinyi A4, Otiende YM1, Mungai PG4
1Wildlife Forensics and Genetics Laboratory, Kenya Wildlife Service, Nairobi, Kenya
2Department of Biochemistry & Molecular Biology, Egerton University, Nakuru, Kenya
3Department of Biochemistry & Biotechnology, Kenyatta University, Nairobi, Kenya
4Ecological Monitoring Section, Biodiversity Research and Monitoring Division, Kenya Wildlife Service, Nairobi, Kenya
Correspondence to: Aluoch AM, Wildlife Forensics and Genetics Laboratory, Kenya Wildlife Service, Nairobi, Kenya.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The present study examined two genera of coprophilous fungi: Ascobolus and Pilobolus with the aim of species description using their morphological diversity. Fresh dung samples from wild herbivores were collected in different parts of Nairobi National Park in Kenya and immediately taken to the laboratory for culture by moist chamber method. Isolates studied were obtained from dung of the following animals: white rhino, zebra, waterbuck, impala, Cape buffalo, giraffe, Thomson’s gazelle, dikdik, hare, grant’s hartebeest, hippopotamus and eland. Five species of Ascobolus were studied namely: Ascobolus amoenus, A. bistisii, A. calesco and A. immersus. A possible novel 4-spored Ascobolus species was observed. Three species of Pilobolus were found: Pilobolus crystallinus var. crystallinus, P. heterosporus and P. pullus. The most abundant species were: Ascobolus immersus and Pilobolus crystallinus var. crystallinus while the highest diversity was observed in waterbuck dung samples with a total of five different species.
Keywords:
Ascomycetes, Coprophilous, Wildlife, Zygomycetes
Cite this paper: Aluoch AM, Obonyo MA, Okun DO, Akinyi A, Otiende YM, Mungai PG, Morphological Diversity of Ascobolus and Pilobolus Fungi from Wild Herbivore Dung in Nairobi National Park, Kenya, Journal of Microbiology Research, Vol. 5 No. 4, 2015, pp. 134-141. doi: 10.5923/j.microbiology.20150504.03.
1. Introduction
Coprophilous fungi are saprobic thus an important part of the wildlife ecosystem since they aid in recycling nutrients in animal dung [1]. In addition, they are thought to influence digestive efficiency of animals as well as being part of nutrition for certain arthropods living in herbivore dung [2].Diversity studies on coprophilous fungi are essential since they give an indication of the state of the environment as they reveal the extent to which environmental stressors contribute to degradation of the environment [3]. High fungi species diversity demonstrates an undisturbed ecosystem that is suitable for the flourishing of wildlife [4]. According to Tibuhwa et al. (2011), macro fungi diversity decreases in areas where agriculture is practiced as opposed to protected wildlife areas with little external environmental stress factors. Species richness is important as an index of the community structure which is useful for conservation [5].The members of fungi in the genus Pilobolus belong to the class Zygomycetes and can be identified through their characteristic sporangiophores that have a swollen extension referred to as collumelae and a sporangium that host the spores at the top [6]. They are observed within two or three days of incubation of dung at room temperature with alternate periods of natural light and darkness [7]. Pilobolus are naturally obligate coprophilous species and only grow on dung materials [8]. They are attached to the dung by a swollen trophocyst which is semi immersed in the dung [9]. These trophocysts are normally ovoid to globose while the rhizoidal extensions are long and cylindrical [9]. Pilobolus have straight unbranched sporangiophores which grow towards light [9]. The sporangiophores have orange pigments at the base and near the subsporangial vesicles. The sporangia are hemispherical in shape with resistant walls and contain the spores which are spherical or ellipsoid depending on species [9].The genus Ascobolus belongs to the class Ascomycetes which consists of a group of phototropic fungi which release their ascospores towards light. In general, members of this genus take longer than Pilobolus to fruit (and is observed after about seven to twenty days following incubation) compared to those of Pilobolus [7], [10], [11]. The fruit body of Ascobolus has a disk and a receptacle which vary in shape from: perithecoid, pyriform, cylindrical, cupulate, scutullate, discoid and lenticular to pulvinate [12]. They have characteristic thick walled spores that are contained in asci. The ascospores are normally single celled, eight within each asci, colored and can be smooth or rough [13].Previous studies on the genus Ascobolus undertaken on wildlife dung in Kenya indicate that there is high species richness [14]. However, there are no specific studies on Pilobolus species in Kenya. Pilobolus is especially important due to its role in transmission of bronchitis in animals as a vector for lungworms [15]. Therefore, it is necessary to establish which species are present in the park and their abundance.This study was focused on species diversity of coprophilous Ascobolus and Pilobolus from wild herbivores with the aim of identifying the different species present in herbivore dung. The results of this study will provide reference for future biodiversity studies and ecological monitoring of the Nairobi National Park ecosystem.
2. Materials & Methods
Wildlife herbivore dung was collected from Nairobi National Park in Kenya. The park is situated approximately 7 km from Nairobi city centre with a savannah ecosystem comprising of scattered acacia and open grass plains. The park covers an area of 117km2 with central coordinates 1°22′24″S, 36°51′32″E. Wild herbivore dung samples were randomly collected from different locations of the park. During collection, all sites were marked using GPS and used to generate a map of sample collection as shown (Fig. 1). Fresh dung samples were collected and placed in paper envelopes and labelled. The following information was recorded for each sample: vegetation type in the surrounding, and animal species voiding the dung.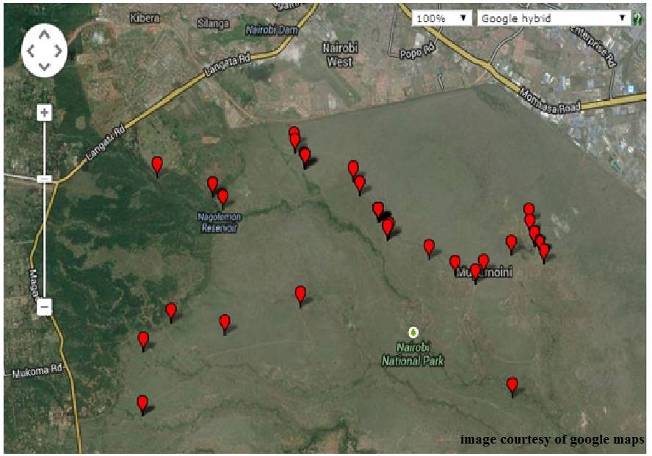 | Figure 1. Map of Nairobi National Park showing sample collection points |
Upon collection, the herbivore dung was transported to the KWS laboratory. Once in the laboratory, the dung samples were incubated in moist chamber under natural conditions of light and temperature for up to twenty one days. This was achieved by placing the dung in labelled 100mm by 15mm glass Petri dishes lined with Whatman cellulose filter paper. Those that were dry were moistened with distilled water. The Petri dishes were positioned near a window to permit natural light to induce sporulation (van Brummelen, 1967). The set up was monitored daily for sporulation under a stereomicroscope (LEICA Microsystems®). Upon sporulation, fruiting bodies were picked using a sharp sterile needle and placed on microscope slides with a drop of water and covered in a cover slip then examined under a compound microscope. The morphological features such as shape, size, colour, height and width were observed and measured for each fruiting body using LAS Suite software®. In order to obtain an average spore length 10 spores were measured from each fruiting body. Apart from measurements, the features were also observed and unique characteristics recorded for the morphological descriptions. The observed morphological features were used to identify the species following keys developed by van Brummelen (1967) and Viriato (2008).In order to get the diversity index for the fungi observed in the dung samples, Shannon-weinner index and Simpson diversity index were calculated [16]. Chao’s species richness estimation was used to calculate the expected highest species abundance [5], [17].
3. Results
3.1. Isolates and Morphology
Ascobolus amoenus Oudem., Hedwigia 21: no. 11 (1882) (Fig. 2A-F).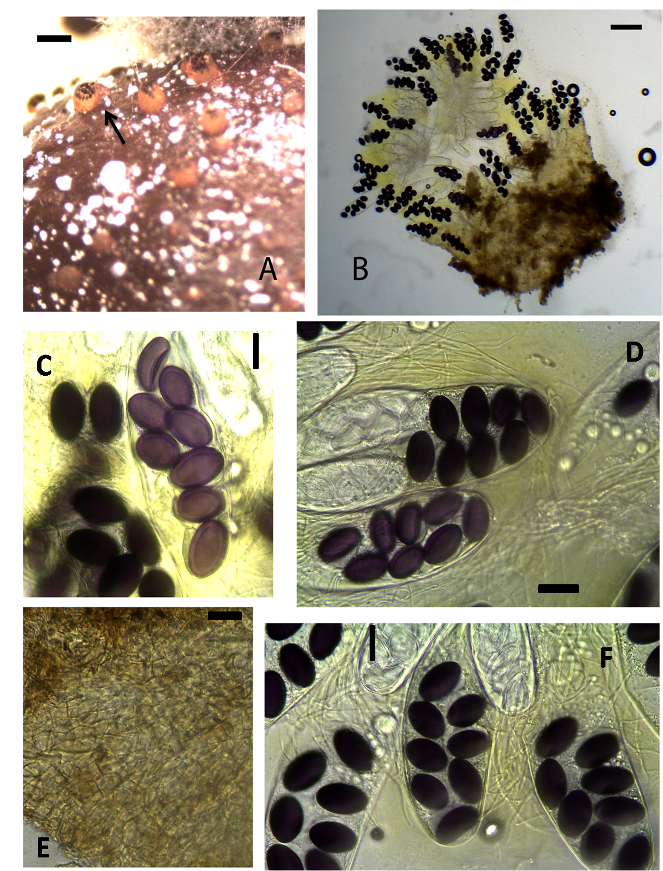 | Figure 2. Ascobolus amoenus (KWSNNP005-2013). A. Ascomata on substrate (arrow). B. Specimen squashed in glycerol. C. 8-spored ascus. D. Asci. E. Ascomatal wall. F. asci and paraphyses. Scale bars: A = 2000 μm, B = 500 μm, C = 20 μm, D = 50 μm, E = 50 μm, F = 50 μm |
Ascomata apothecioid, solitary or gregarious, semi-immersed, 500–600 μm high, 600–700 μm diam. Receptacle at first closed then irregularly opening at the top, cupulate, brown. Disc flat to convex, greenish yellow, numerous tips of ripe asci protruding and dotting the hymenium. Hypothecium thin discontinuous and composed of isodiametric and oblong cells 4–6 μm. Medullary excipulum of textura globulosa thin cells. Ectal excipulum of textura globulosa-angularis brownish cells 10–20 × 6–10 μm. Paraphyses filiform, hyaline, tips rarely inflated, embedded in greenish-yellow mucus 2–4 μm broad. Asci 180–245 × 50–60 μm, 8-spored, narrowly clavate, rounded and curved with walls that characteristically stain blue in Melzer’s reagent. Ascospores 30–40 × 21–22 μm, biseriate, ellipsoidal, violet, and finally brownish, with thin gelatinous sheath.Material examinedKENYA, Nairobi County, incubated for seven to fourteen days, Nairobi National Park, GPS S1°35’45.11” E36°85’70.55”, altitude 1647 m, waterbuck, 23 July 2013, P. Mungai, KWSNNP005-2013; Nairobi National Park, Nairobi County, GPS S1°35’91.55” E36°84’48.32”, altitude 1649m, impala, 23 July 2013, P. Mungai, KWSNNP007-2013; Nairobi National Park, Nairobi County, GPS S1°35’42.28” E36°85’68.92”, altitude 1650m, hartebeest, 26 August 2013, P. Mungai, KWSNNP020-2013; Nairobi National Park, Nairobi County, GPS S1°34’77.08” E36°85’17.54”, altitude 1648m, Thomson’s gazelle, 26 August 2013, P. Mungai, KWSNNP012-2013.NotesAscobolus amoenus sect Dasyobolus [18] is closely similar to A. Elegans [19] but it can be differentiated by its smaller ascospores. This collection differs from that described by Oudemans (1882) in the size of the asci. The latter has asci with smaller diameter of about 35–40 μm. The ascospores were observed to have double walls.Ascobolus bistisii Gamundí & Ranalli, Nova Hedwigia 10: 347 (1966) [1965] (Fig. 3A-F)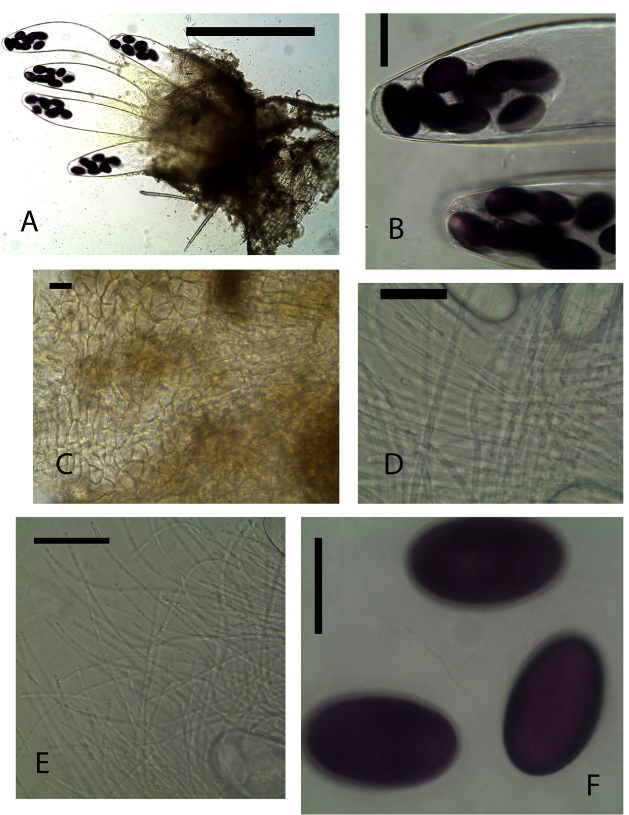 | Figure 3. Ascobolus bistisii (KWSNNP001-2013). A. Ascoma squashed in water. B. Ascospores at the tip of asci. C. Ascomatal wall. D-E. Paraphyses. F. Ascospores. Scale bars: A = 200 μm, B = 50μm, C = 20μm, D = 20μm, E = 20μm, F = 20μm |
Ascomata cleistothecioid early stages, hymenium exposed later on, gregarious, superficial or semi-immersed, 600–700 μm high 400–500 μm diameter. Receptacle, subglobose, brown, dotted with few protruding, finger-like asci, barrel shaped, widest at equatorial part, with a hardly differentiated margin. Disc convex, light yellow to brown. Hypothecium very thin of isodiametric cells. Medullary excipulum of textura angularis cells 5–10 × 6–20 μm. Ectal excipulum of textura angularis 15–20 × 7–8 μm. Paraphyses cylindric-filiform, with tips not inflated, embedded in clear mucus, numerous septae, 3–4 μm. Asci 400–500 × 100–105 μm, 8-spored, broadly clavate-cylindrical, operculate with dome-shaped apex 30–40 μm wide, wall weakly amyloid. Ascospores 53–58 × 30–33 μm, irregularly biseriate, ellipsoidal, rounded at the ends, purple, irregularly distributed at the end of the ascus surrounded by thin gelatinous sheath.Material examinedKENYA, Nairobi National Park, Nairobi County, Specimens, dung incubate for 7-9 days. GPS S 1°35’43.02” E 36°85’68.79”, altitude 1657 m, white rhino, 23 July 2013, P. Mungai, KWSNNP001-2013; Nairobi National Park, Nairobi County, GPS S 1°35’75.45” E 36°84’63.72”, altitude 1649 m, zebra, 23 July 2013, P. Mungai, KWSNNP006-2013; Nairobi National Park, Nairobi County, GPS S 1°35’43.02” E 36°85’68.79”, altitude 1657 m, waterbuck, 23 July 2013, P. Mungai, KWSNNP003-2013; Nairobi National Park, Nairobi County, GPS S1°34’77.08” E36°85’17.54”, altitude 1648 m, Thomson’s gazelle, 26 August 2013, P. Mungai, KWSNNP012-2013; Nairobi National Park, Nairobi County GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, dikdik, 10 January 2014, A. Aluoch, KWSNNP024-2014.NotesAscobolus bistisii Sect. Dasyobolus [20] is similar to Ascobolus immersus in many ways morphologically. However, this species has regularly ellipsoid ascospores while those of A. immersus [21] are subcylindrical with rounded ends. This species is quite common in Kenya wildlife dung as observed in this study.Ascobolus calesco A.E. Bell & Mahoney, Fungal Planet, no. 11-21: 21: [2] (2007). (Fig. 4A-F)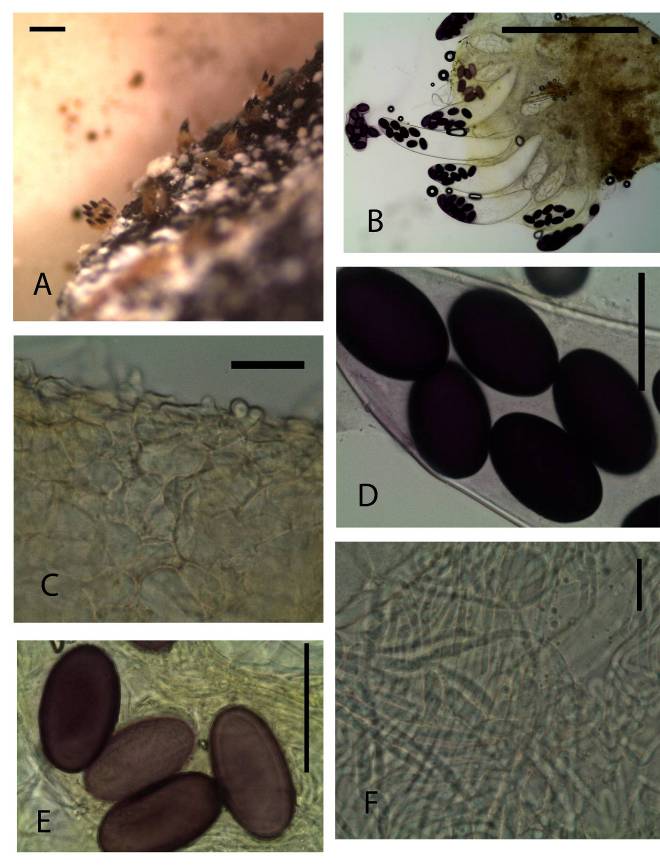 | Figure 4. Ascobolus calesco (KWSNNP020-2013). A. Ascomata on substrate. B. Ascoma squashed in lactic acid. C. Ascomatal wall. D-E Ascospores. F Paraphyses. Scale bars: A = 1000 μm, B = 500 μm, C = 20 μm, D = 20 μm, E = 20 μm, F = 20μm |
Ascomata apothecioid, scattered or gregarious, semi-immersed 800 μm high, 700 μm diam. Receptacle deep yellow to yellowish-brown, subglobose, barrel shaped, margin not differentiated. Disc globular flat ripe asci protruding above the hymenium. Hypothecium not well differentiated from Medullary excipulum. Ectal excipulum of textura angularis brown cells, 14–21 × 7–11 μm. Paraphyses filiform, hyaline, simple or sparingly branched at the base, septate, exceeding asci, 2–4 μm broad, tips not swollen, embedded in greenish-yellow mucus. Asci 600 × 100 μm, 8-spored, unitunicate Ascospores 48–57 × 27–33 μm, biseriate, single-celled, ellipsoidal, purple. Gelatinous sheath hyaline, surrounding each ascospore.Material examinedKENYA, Nairobi National Park, Nairobi County, one specimen, dung incubated for seven to fourteen days, GPS S 1°35’42.28” E 36°85’68.92”, altitude 1650 m, hartebeest, 26th August 2013, P. Mungai, KWSNNP020-2013.NotesThe Kenya Ascobolus calesco Sect. Dasyobolus described here agrees with the description of A calesco as described by Bell and Mahoney (2007) [22].Ascobolus immersus Pers., Neues Mag. Bot. 1: 115 (1794) (Fig. 5A-F).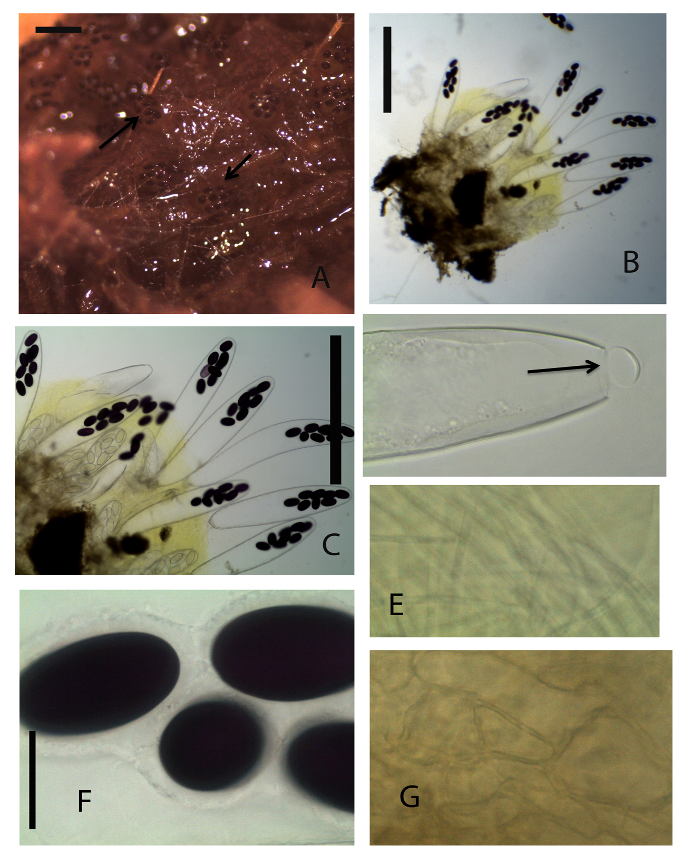 | Figure 5. Ascobolus immersus (KWSNNP001-2013). A. Ascomata on substrate (arrows). B. Ascoma squashed in water. C. 8-spored mature asci. D. Open operculum (arrow). E. Paraphyses. F. Mature ascopores surrounded by gelatinous sheath. G. Ascomatal wall. Scale bars: A=1000μm, B= 500μm, C=200μm, D=50μm, E=20μm, F=20μm, G=20μm |
Ascomata clestothecoid at first, gregarious or scattered, immersed or superficial, sessile, 700–1000 μm high, 600–800 μm diam. Receptacle deep yellow to yellowish-brown or greenish-brown, subglobose, margin not differentiated. Disc flat or convex without margin, shiny, a few ripe asci protruding above the hymenium. Hypothecium very thin, of isodiametric cells. Medullary excipulum thin, of textura globulosa or angularis hyaline cells. Ectal excipulum of somewhat horizontally elongated textura angularis yellowish-brown thick walled cells, 22–43 × 11–17μm. Paraphyses filiform, simple or sparingly branched at the base, septate, exceeding asci 2–3.5 μm broad, embedded in greenish-yellow mucus. Asci broadly clavate 460–675 × 95–115 μm, 8-spored, unitunicate broadly clavate to sacciform rounded above. Ascospores 55–60 × 30–35 μm, biseriate, single-celled, subcylindrical, ends markedly rounded, violet becoming purple at maturity. Gelatinous sheath on each spore, hyaline, broader on sides and narrow on polar region.Material examinedKENYA, Nairobi National Park, Nairobi County, seven specimens, dung incubated for 7-15 days, GPS S 1°34’27.03” E 36°82’22.23”, altitude 1657 m, buffalo, 23 July 2013 P. Mungai, KWSNNP010-2013; Nairobi National Park, Nairobi County, GPS S 1°35’43.02” E 36°85’68.79”, altitude 1657 m, white rhino, 23 July 2013, P. Mungai, KWSNNP001-2013; Nairobi National Park, Nairobi County, GPS S 1°35’45.11” E 36°85’70.55”, altitude 1647 m, zebra, 23 July 2013, P. Mungai, KWSNNP002-2013; Nairobi National Park, Nairobi County, GPS S 1°35’43.02” E 36°85’68.79”, altitude 1657 m, waterbuck, 23 July 2013, P. Mungai, KWSNNP003-2013; Nairobi National Park, Nairobi County, GPS S 1°35’42.28” E 36°85’68.92”, altitude 1650 m, hartebeest, 26 August 2013, P. Mungai, KWSNNP020-2013; Nairobi National Park, Nairobi County, GPS S1°34’77.08” E36.85’17.54”, altitude 1648 m, Thomson’s gazelle, 26 August 2013, P. Mungai, KWSNNP012-2013; Nairobi National Park, Nairobi County GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, white rhino, 10 January 2014, A. Aluoch, KWSNNP031-2014; Nairobi National Park, Nairobi County GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, hare, 10 January 2014, A. Aluoch, KWSNNP026-2014; Nairobi National Park, Nairobi County GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, eland, 10 January 2014, A. Aluoch, KWSNNP037-2014; Nairobi National Park, Nairobi County GPS S1°84’64.98” E37°02’65.83”, altitude 1619 m, zebra, 14 January 2014, A. Aluoch, KWSNNP039-2014.NotesAscobolus immersus Sect. Dasyobolus [21] is quite common in dung from Kenya wildlife and domestic herbivore dung. It is easily distinguished from other members of the Ascobolus genus by its characteristically large ascospores. Each spore is surrounded by a gelatinous sheath. They can grow immersed on soft surfaces while growing on the surface of more dense surfaces.Ascobolus sp (KWSNNP020-2013 hartebeest dung) (Fig. 6A-G)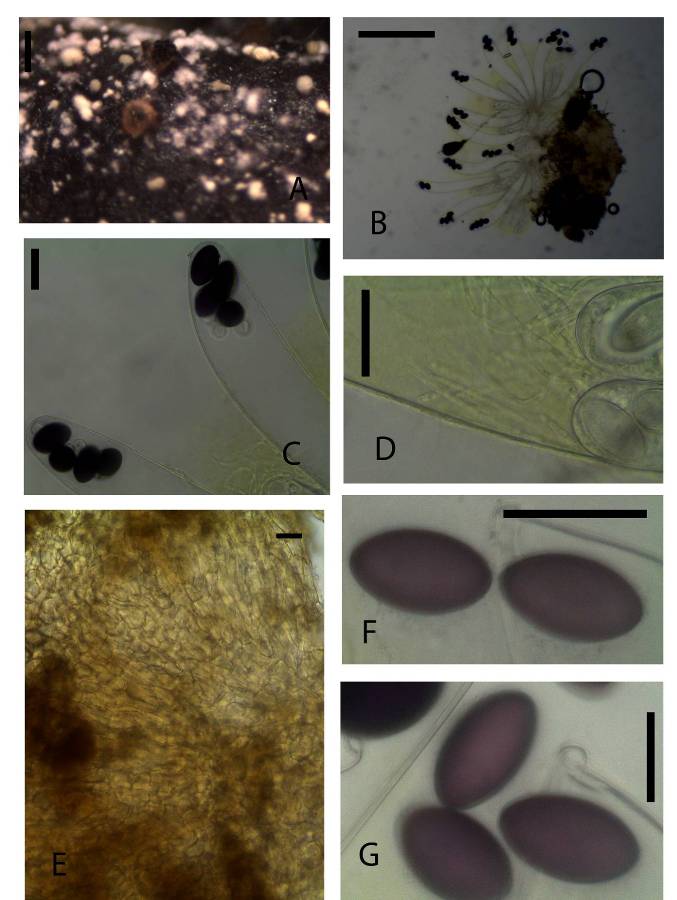 | Figure 6. 4-spored Ascobolus sp. (KWSNNP020-2013). A. Ascomata on substrate. B. Ascoma squashed in water. C. 4-spored asci. D. Paraphyses. E. Ascomatal wall. F-G. Ascospores. Scale bars: A = 1000 μm, B = 500 μm, C = 50 μm, D = 20 μm, E = 20 μm, F = 20μm, G = 20μm |
Ascomata apothecioid, scattered, immersed, 380 μm high, 540 μm diam. Receptacle deep brown, subglobose, margin not differentiated. Disc flat ripe asci protruding above the hymenium. Hypothecium thin with globose cells. Medullary excipulum of yellowish brown cells of various thickness. Ectal excipulum of textura angularis yellowish-brown cells, 10–25 × 10–12 μm. Paraphyses filiform, tips not swollen, embedded in greenish-yellow mucus. Asci 365–400 × 60–65 μm, 4-spored sacciform, rounded above. Ascospores 35–45 × 20–25 μm, uniseriate, single-celled, subcylindrical, ends markedly rounded, purple Gelatinous sheath absent.Material examinedKENYA, Nairobi National Park, Nairobi County, hartebeest 26th August 2013 dung incubated for seven days, GPS S 1°35 E 36°86, altitude 1650 m, P. Mungai, KWSNNP020-2013.NotesThis species differs from those described earlier due to the fact that it contained four ascospores in each ascus. The ascospores are seen clustered on one end of the ascus.Pilobolus crystallinus (F. H. Wigg.) Tode var. crystallinus, Schrift. Berl. Gesell. Naturf. Freunde 5: 47 (1784) (Fig. 7 A-F) | Figure 7. Pilobolus crystallinus var. crystallinus (KWSNNP004-2013). A. Pilobolus on substrate. B. Sporangium and Columella. C. Trophocyst and rhizoidal extension. D. Zygospores. E. Trophocyst. F. Zygospores. Scale bars: A = 2000 µm, B = 500 µm, C = 200 µm, D = 50 µm, E = 50 µm, F = 50 µm |
Trophocyst subglobose, 370×360 µm with rhizoidal extension up to 980 µm, yellowish pigmentation. Sporangiophore long-cylindrical, unbranched, phototrophic, 4mm×100 µm. Sporangial wall black, hemispherical to ovoid 480×250 µm. Columella smooth walled. Subsporangial vesicle smooth walled a little orange pigmentation elliptical 700×530 µm. Spores yellow, grainy content, smooth wall, ellipsoid 8×5 µm.Material examinedKENYA, Nairobi National Park, Nairobi County, incubated for four to seven days, GPS S 1°35’45.11” E 36°85’50.89”, altitude 1646 m, zebra, 23 July 2013, P. Mungai, KWSNNP004-2013; Nairobi National Park, Nairobi County, incubated for three to seven days, GPS S 1°34’50.02” E 36°84’82.34”, altitude 1649 m, buffalo, 23 July 2013, P. Mungai, KWSNNP009-2013; Nairobi National Park, Nairobi County GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, Grant’s gazelle, 10 January 2014, A. Aluoch, KWSNNP027-2014; Nairobi National Park, Nairobi County GPS S1°84’64.98” E37°02’65.83”, altitude 1619 m, zebra, 14 January 2014, A. Aluoch, KWSNNP032-2014.NotesOur specimen closely agrees with the Pilobolus crystallinus var. crystallinus described in the original diagnosis by F. H. Wigg. Tode Schrift. Berl. Gesell. Naturf. Freunde 5: 47 (1784) [23]. Some specimens had spore sizes differing slightly from those described earlier. Pilobolus crystallinus var. crystallinus differs from Pilobolus crystallinus var. kleinii by having pale yellow spores while those of the latter are bright yellow.Pilobolus pullus Massee, Bull. Misc. Inf., Kew: 160 (1901) (Fig. 8A-F) | Figure 8. Pilobolus pullus (KWSNNP009-2013). A. Pilobolus on substrate. B. Pilobolus squashed in glycerol. C. Columella and sporangiophore. D. Sporangium. E-F. Zygospores. Scale bars: A = 2000 µm, B = 500 µm, C = 200 µm, D =50 µm, E = 50 µm, F = 20 µm |
Trophocysts ovoid to globose, hyaline 180 µm diameter. Sporangiophore long-cylindrical, 720×90 μm. Sporangia black, hemispherical 270×140 μm. Columella with smooth walls. Subsporangial vesicle with smooth wall hyaline, little pigmentation, ovoid, 370×200 μm. Zygospores 9×5 μm yellow, homogenous content, subcylindrical.Material examinedKENYA, Nairobi National Park, Nairobi County, incubated for three to seven days, GPS S 1°34’50.02” E 36°84’82.34”, altitude 1649 m, buffalo, 23 July 2013, P. Mungai, KWSNNP009-2013; Nairobi National Park, Nairobi County, incubated for four to seven days, GPS S 1°35’45.11” E 36°85’50.89”, altitude 1646 m, zebra, 23 July 2013, P. Mungai, KWSNNP004-2013; Nairobi National Park, Nairobi County GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, giraffe, 10 January 2014, A. Aluoch, KWSNNP034-2014.NotesThe isolated material agrees with the original diagnosis [24]. However, the rhizoidal extensions for some of the identified species were longer than the 300 µm of the described species.Pilobolus heterosporus Palla, Öst. bot. Z. 50: 349 (1900) (Fig. 9A-F)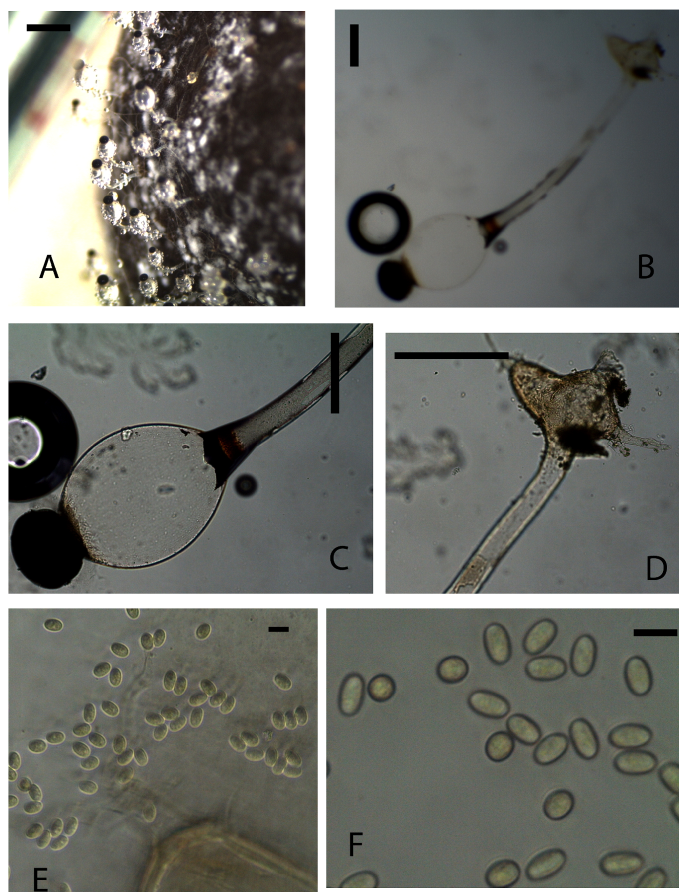 | Figure 9. Fig. 9. Pilobolus heterosporus (KWSNNP021-2013). A. Pilobolus on substrate. B. Pilobolus squashed in glycerol. C. Columella and sporangiophore. D. Rhizoidal extension. E-F. Zygospores. Scale bars: A = 2000 µm, B = 500 µm, C = 200 µm, D =50 µm, E = 50 µm, F = 20 µm |
Trophocysts ovoid to globose, short ellipsoid 200.27×280.46 µm with rhizoidal extensions little pigmentation. Sporangiophores long cylindrical, 1.5mm × 69.23 µm. Sporangia black, hemispherical to ovoid 169.7×212.29 µm, resistant wall. Columella conical, little pigmented. Subsporangial vesicles ovoid and ellipsoid, 412.31×344.65µm. Zygospores 5.6-10.08×5.15-6.1 µm yellowish grainy content, ovoid, cylindrical.Material examinedKENYA, Nairobi National Park, Nairobi County, incubated for three to seven days, GPS S1°85’07.59” E37°02’58.18”, altitude 1658 m, dikdik, 10 January 2014 A. Aluoch, KWSNNP024-2014.NotesThis species shows similarities to those described by Viriato (2007). The described species is differentiated from the others due to the different shaped irregular zygospores with grainy content.
3.2. Diversity Index
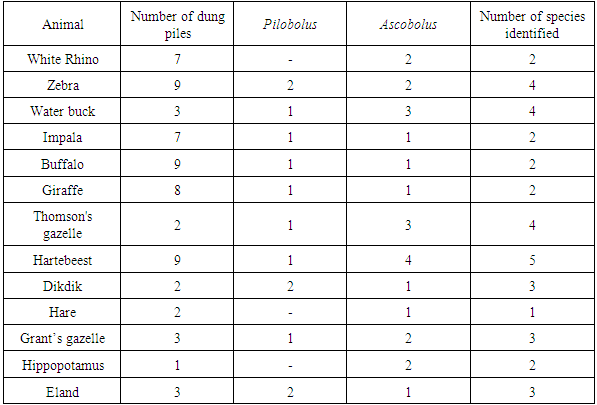
There were a total of 8 species reported from 13 different animal hosts (table 1). Three from the genus Pilobolus and 5 from the genus Ascobolus. The highest recorded species was Ascobolus immersus followed by Pilobolus crystallinus. These were abundant in a large variety of the animal dung samples collected. However, since the number of samples collected for each animal species was not equal, the number of coprophilous fungi species found in each dung sample was used to calculate diversity index. There was no growth of any of the two genera of interest in this study in two rhino, two impala, three buffalo, and six giraffe dung samples. The maximum fungi species of interest in this study was observed in waterbuck dung which had 3 Ascobolus species and 2 Pilobolus species.Table 1. Host animals and fungal species described in each
 |
| |
|
Across the 68 dung samples collected, there were 8 different coprophilous fungal species recorded. Since most of them were rare, Chao’s formula to obtain the estimated richness. This gave an estimated richness as 15 different species per dung sample compared to the observed richness of 5 species.
4. Discussion
According to the findings of the current study, five species from the genus Ascobolus were identified namely: A. amoenus, A. bistisii¸ A. calesco, A. immersus and fifth putatively new species (a species with 4-spored asci) that has never been described to the best of our knowledge. The most abundant species from the animal dung samples was A. immersus (28.9%). In addition, there were three Pilobolus species identified, that is: P. crystallinus var. crystallinus, P. heterosporus and P. pullus. Pilobolus crystallinus var crystallinus (25.2%) was the most abundant species in this genus.Fungi from the genus Pilobolus were observed to grow and die out within the first week of incubation in a moist chamber. However, for some samples, there was still new growth of Pilobolus even after ten days of incubation. This could be attributed to the fact that some of the spores dispersed within the plates were falling on the dung and growing again. This may mean that Pilobolus genus do not require passage in the animal gut or treatment to sporulate.The observed diversity and expected diversity indicate that there is high species richness of coprophilous fungi in Nairobi National Park. This is likely to indicate that even though the park is located near the capital city, surrounded by upcoming industrial areas as well as increasing human settlements, the park ecosystem itself is relatively well preserved. This is comparable to the work of Tibuwa et al. (2011) who studied macro fungi diversity in Serengeti-Mara ecosystem and reported high diversity in woodland and grassland areas as compared to areas with human settlements. The theory that there is little interference in the ecosystem is also supported by the fact that there was no outright dominant species that are adapted to altered environment conditions since the difference between the abundance of the observed species was not very significant [3], [25].Future studies on morphological diversity of these coprophilous fungi genera and comparison with the results of this study will aid in monitoring the changes in the park ecosystem.
ACKNOWLEDGEMENTS
This work was made possible by the generous support of the Kenya Wildlife Service technical and scientific staff.
References
| [1] | M. J. Richardson, “Diversity and occurrence of coprophilous fungi,” vol. 105, no. April, pp. 387–402, 2001. |
| [2] | D. T. Wicklow and K. Angel, A Preliminary Survey of the Coprophilous Fungi from a Semi-arid Grassland in Colorado. Natural Resource Ecology Laboratory, Colorado State University, 1974. |
| [3] | C. Ebersohn and A. Eicker, “Coprophilous fungal species composition and species diversity on various dung substrates of African game animals,” Bot. Bull. Acad. Sin., vol. 33, pp. 85–95, 1992. |
| [4] | D. D. Tibuhwa, M. N. Muchane, C. W. Masiga, C. Mugoya, and M. Muchai, “An Inventory of Macro-fungi and their Diversity in the Serengeti-Masai Mara Ecosystem, Tanzania and Kenya.,” J. Biol. Sci., vol. 11, no. 6, 2011. |
| [5] | N. J. Gotelli and R. K. Colwell, “Estimating species richness,” Biol. Divers. Front. Meas. Assess., pp. 39–54, 2011. |
| [6] | B. Kendrick, The fifth kingdom. Focus Publishing, R. Pullins Co., Newburyport, Massachusetts, USA, 2000. |
| [7] | A. Bell, Dung fungi: an illustrated guide to coprophilous fungi in New Zealand. Wellington (MO): Victoria University Press, 1983. |
| [8] | J. C. Krug, G. L. Benny, and H. W. Keller, “Coprophilous Fungi,” in Biodiversity of fungi, Mueller GM, Bills GF, Foster MS. Academci Burlington, 2004, pp. 468–499. |
| [9] | A. Viriato, “Pilobolus species found on herbivore dung from the Sao Paulo Zoological Park, Brazil,” Acta Bot. Brasilica, vol. 22, pp. 614–620, 2008. |
| [10] | O. Piasai, L. Manoch, and others, “Coprophilous ascomycetes from Phu Luang Wildlife Sanctuary and Khao Yai National Park in Thailand,” Kasetsart J. (National Sci., vol. 43, pp. 34–40, 2009. |
| [11] | P. Mungai, K. D. Hyde, L. Cai, J. Njogu, and K. Chukeatirote, “Coprophilous ascomycetes of northern Thailand,” Curr. Res. Environ. Appl. Mycol., vol. 1, pp. 135–159, 2011. |
| [12] | J. A. van Brummelen, “A World-monograph of the genera Ascobolus and Saccobolus. Persoonia Suppl. 1, 1-260.,” Persoonia, vol. 11, 1967. |
| [13] | A. Bell, An illustrated guide to the coprophilous Ascomycetes of Australia, Fungal Bio. Utrecht, The Netherlands: CBS Biodiversity Series 3, 2005. |
| [14] | P. G. Mungai, J. G. Njogu, E. Chukeatirote, and K. D. Hyde, “Studies of coprophilous ascomycetes in Kenya. Ascobolus species from wildlife dung,” Curr. Res. Appl. Environ. Mycol., vol. 2, no. 1, pp. 1–16, 2012. |
| [15] | R. Jorgensen, H. Ronne, C. Helsted, and I. AR, “Spread of infective Dictyocaulus viviparus larvae in pasture and to grazing cattle: Experimental evidence of the role of Pilobolus fungi,” Vet. Parasitol., vol. 10, no. 4, pp. 331–339, 1982. |
| [16] | A. E. Magurran, Measuring biological diversity. Taylor & Francis, 2004. |
| [17] | A. Chao, “Nonparametric estimation of the number of classes in a population,” Scand. J. Stat., pp. 265–270, 1984. |
| [18] | Oudemans, “Ascobolus amoenus,” Hedwigia, vol. 21, p. 11, 1882. |
| [19] | J. Klein, “Ascobolus elegans,” Verh. zool.-bot. Ges. Wien, vol. 20, p. 566, 1870. |
| [20] | Gamundi and Ranalli, “Ascobolus bistisii,” Nov. Hedwigia, vol. 10, p. 347, 1966. |
| [21] | Persoonia, “Ascobolus immersus,” Neues Mag. für die Bot., vol. 1, p. 115, 1794. |
| [22] | A. E. Bell and D. P. Mahoney, “Ascobolus calesco sp. nov,” Fungal Planet, vol. 21, 2007. |
| [23] | F. H. Wigg, “Pilobolus crystallinus,” Schr. naturf. Fr. Berlin, vol. 5, p. 96, 1784. |
| [24] | G. E. Massee, “Fungi Exotici, III,” Bull. Misc. Inf. (Royal Bot. Gard. Kew), p. 160, 1901. |
| [25] | E. P. Odum, “Trends expected in stressed ecosystems,” Bioscience, vol. 35, no. 7, pp. 419–422, 1985. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML







