-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(4): 128-133
doi:10.5923/j.microbiology.20150504.02
Deactivation of Uropathogenic Proteus Bacterial Toxin by Polyphenols of Tamarindus indica Bark: A Robust Inhibition of Hemolysis
Aktar Uzzaman Chouduri , Mamunur Roshid , Najem Uddin , Abdul Wadud
Department of Pharmacy, University of Rajshahi, Rajshahi, Bangladesh
Correspondence to: Aktar Uzzaman Chouduri , Department of Pharmacy, University of Rajshahi, Rajshahi, Bangladesh.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
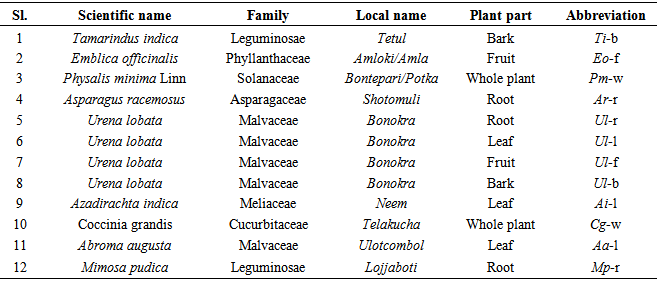
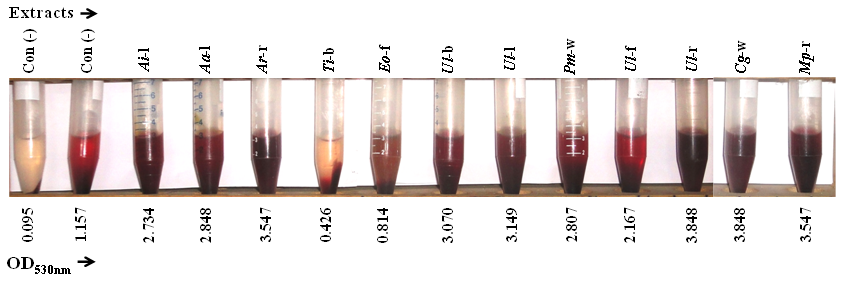
Increasing evidence of multi-antibiotic resistance in bacterial pathogens necessitates medicinal plants as an alternate therapy of infection control and management. Tamarindus indica L. is widely investigated but limited information is available on its bark (Ti-b). Ethanol extract of twelve medicinal plants including Ti-b were investigated for their activities to inhibit hemolysis caused by multi-drug resistant uropathogen, Proteus. Proteus vulgaris among other Proteus species was found as highly hemolytic. Hemolysis caused by cell free hemolysins of P. vulgaris was predominant (75%) over that of cell-associated and intracellular hemolysins. This toxin mediated hemolysis of human erythrocyte cells was potentially inhibited by Ti-b extract although its antimicrobial activity was too low. Bioactive inhibitor in Ti-b extract was detected as polyphenol, which enabled to deactivate the active site of hemolysins and thereby protected blood cells from toxin mediated hemolysis.
Keywords: Ethanol extract, Hemolysin inhibitor, Polyphenol, Proteus valgaris, Tamarindus indica bark
Cite this paper: Aktar Uzzaman Chouduri , Mamunur Roshid , Najem Uddin , Abdul Wadud , Deactivation of Uropathogenic Proteus Bacterial Toxin by Polyphenols of Tamarindus indica Bark: A Robust Inhibition of Hemolysis, Journal of Microbiology Research, Vol. 5 No. 4, 2015, pp. 128-133. doi: 10.5923/j.microbiology.20150504.02.
Article Outline
1. Introduction
- Proteus bacteria are the third most common cause (after Escherichia coli and Klebsiella pneumoniae) of UTI, but it is the most serious because it causes damage such as catheter blockage, stone formation in kidney and urinary bladder, cystitis, pyelonephritis, and bacteremia [1–2]. Infections caused by Proteus bacteria are characterized as long term which is difficult to treat and can often lead to death due to their capacity of urease-mediated urea hydrolysis causing tissue necrosis and inflammation at the infection site, so that the pathogen is inaccessible to antibiotics [3–5]. Production of cytotoxic hemolysins is common in both Gram-positive and -negative pathogenic bacteria including Proteus, but its extent in inter-species and intra-species varies greatly [6]. The hemolytic activity of Proteus bacteria is associated to hemolysins, HpmA and HlyA. Especially the predominant hemolysin HpmA is responsible for tissue damage which is activated when its N-terminal peptide is cleaved [7]. Conventional systems of herbal medicine have been using from ancient times. Medicinal plants especially herbs have been the principal source of most of the drugs. Now-a-days about 70% of the world population is depending on medicinal herbs. Medicinal plants contain so many chemical compounds which are the major source of therapeutic agents to cure human diseases [8]. For a long period of time, medicinal plants have been the valuable source of natural products for maintaining human health, especially in the last decade, with more intensive studies for natural therapies. The use of phytochemicals for pharmaceutical purposes has been gradually increased. According to World Health Organization the medicinal plants could be the best source to obtain a variety of drugs. Approximately 80% people in developed countries use traditional medicine, which has compounds derived from medicinal plants. Therefore, such plants should be investigated to better understand their properties, safety and efficiency [9]. The use of plant extracts and phytochemicals, both with known antimicrobial properties, can be of great significance in therapeutic treatments. In the last few years, a number of studies have been conducted in different countries to prove such efficiency [10–14]. Many plants have been used for their antimicrobial traits, which are due to compounds synthesized in the secondary metabolism of the plant. The antimicrobial properties of medicinal plants have been investigated by many investigators worldwide, especially in Indian region. Thirty one medicinal plant species have been reported by traditional healers as being used for UTIs, including leucorrhea, frequent or infrequent urination, cloudy urination, and burning sensations during urination in Bangladesh [15].We isolated pathogenic Proteus bacteria from municipal tap water [16] that were multidrug resistant especially to cephalosporin [17], and several pathogenic features of those isolates have already been reported [18–19]. The increasing evidence of antibiotic resistance in bacterial pathogens necessitates medicinal plants as an alternative therapy in restricting the antibiotic resistant infectious organisms. We selected twelve plant parts from nine medicinal plants species having potential antimicrobial properties as enlisted table-1 that are traditionally used as folk medicine for urological disorder [20–25]. Nwodo et al found the significant antimicrobial activities in aqueous and alcoholic extract of Tamarindus indica bark [26]. The aqueous extract of Emblica officinalis fruit pulp is found to be effective against various pathogenic bacteria including Proteus species [27]. This may be due to the presence of certain tanin, alkaloids and phenolic compounds present in the fruit of Emblica officinalis [28]. The present study aimed to investigate the effectiveness of ethanolic extract of test medicinal plants to inhibit the growth and pathogenesis of cephalosporin resistant Proteus bacteria isolated from municipal supplied water.
|
2. Materials and Methods
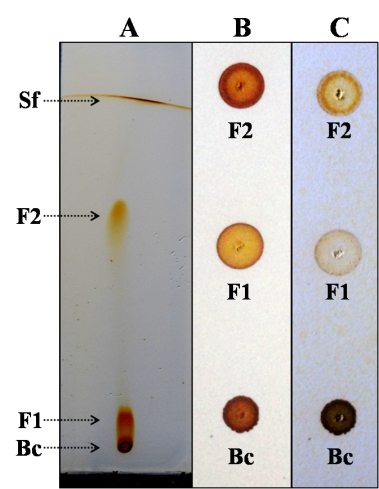
- Bacterial strainsEleven Proteus bacterial strains belonging four species i.e. P. vulgaris (hereafter termed as Pv), P. mirabilis (Pm), P. hauseri (Ph), and P. penneri (Pp) designated as 11(Pv), 661(Pp), 662(Ph), 663(Pp), 664(Pp), 665(Pp), 666(Pp), 667(Pp), 668(Pp), 911(Pm) and 912(Pm) isolated from municipal tap water of Rajshahi City, Bangladesh in previous study have been used [16]. These strains were multidrug resistant to broad spectrum antibiotics and possessed several pathogenic features including swarming motility, urease production, extracellular proteases, biofilm formation as reported earlier [17–19]. Strains stored at −40ºC in Luria-Bertani (LB) broth supplemented with 12% (v/v) glycerol were freshly grown at 37ºC to carry out this study.Plant material Plant parts were collected from the medicinal plant garden of University of Rajshahi and around Rajshahi City area, Bangladesh on November to December 2013, and duly identified by a plant taxonomist, Department of Botany, University of Rajshahi, Bangladesh where a specimen voucher (75/05.07.2008, 32/10.05.2007) was recorded in the department herbarium for future reference. Twelve specimens of nine medicinal plants enlisted in table 1 were air-dried under shade. Once dried, the plant material was ground, extracted by maceration for more than 72 hrs with ethanol, filtered (Paper Whatman No. 3) and the solvent was vacuum evaporated in a Soxhlet apparatus (Rotary Evaporator, RE 300, Bibby Sterilin Ltd, UK). Then solutions were evaporated to dryness and further dilutions were made in the same solvent to obtain the required extract concentrations for the different assays. Human erythrocyte preparation10 ml of human blood obtained from healthy donor in anti-coagulating agent sodium oxalate was centrifuged at 5000 rpm for 5 min, discarded the supernatant, washed three times with normal saline (0.9% NaCl) and the pellet was suspended in physiological PBS buffer (150mM NaCl, 5mM KCl, 10mM PBS, 2.5mM CaCl2, 10mM glucose, pH 7.4) [29].Hemolysis and antihemolysis assay Hemolysin activity was determined as follows, 300 μl of bacterial cells (109 cfu/ml) were incubated for 3 hrs at 37°C with 2.7ml of 3% erythrocytes in physiological PBS buffer, pH 7.4 on water bath with mild shaking. Then, the reaction mixture was centrifuged at 5000 rpm for 10 min, and the released hemoglobin in the supernatant was determined at OD530nm. The results of hemolysis were expressed as percent following the equation (Asam−Acon)×100 / (Amax lysis – Acon). As a positive control complete hemolysis was obtained by adding 0.1% (v/v) Triton X-100 to release hemoglobin into medium [30–31]. As a negative control 0.5 mg/ml dextran was used, this ensured the integrity of erythrocyte cell membrane. Anti-hemolytic activity assay was performed following the same protocol in presence of plant extracts in ethanol. Equivalent concentration of ethanol was maintained in both positive and negative control experiments throughout the study.TLC and chemical nature of bioactive compoundFor compound separation using thin layer chromatography (TLC), 100 µl of plant extract was spotted onto the heat activated TLC plate made in the laboratory. Different solvent systems were used as mobile phase and finally ethylacetate: n-hexane (2:1) was selected and used on the basis of best separation obtained. After separation, TLC plate was undertaken to various phytochemical tests using spray technique and the presence of polyphenolic compound in one bioactive fraction was confirmed by treating the fraction with FeCl3 which turned the color of compounds to black [32–33]. Data analysisFor data processing, the software Microsoft Excel 2007 was used. Results of triplicate experiments were averaged, and means ± standard deviations were calculated.
3. Results and Discussion
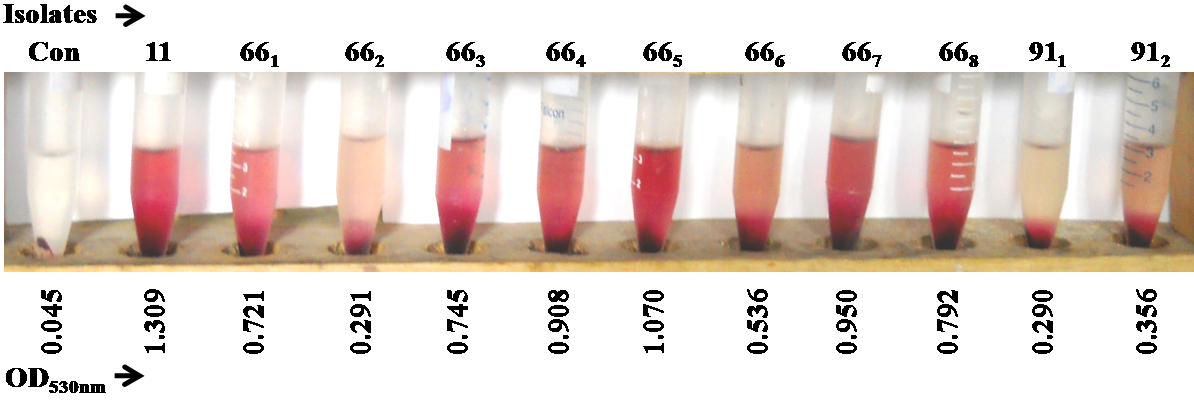
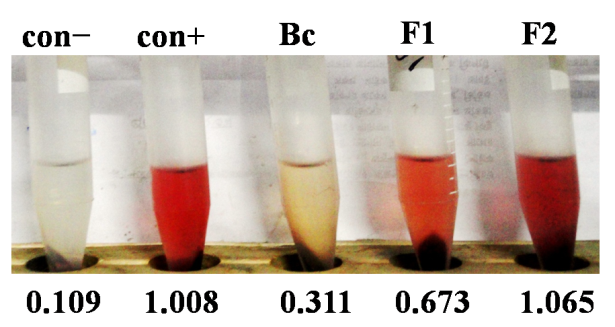
- Hemolytic Proteus isolatesProteus strains isolated in our previous study were screened for their hemolytic activities where human erythrocyte cells were exposed to hemolysin solution i.e. cell free supernatant of Proteus, and the hemolysis was quantified by OD530nm. All test isolates were found to be hemolytic with variable potentials (Figure 1). Isolate 11(Pv) was strongly hemolytic followed by isolates 665(Pp), 667(Pp), 664(Pp), 668(Pp), 663(Pp), 661(Pp), 666(Pp). Isolates 662(Ph), 911(Pm) and 912(Pm) were weakly hemolytic compared to others. It is thought that Pm and Pv are more pathogenic than other Proteus species. However, the results obtained here showed that Pp and Pv are equally pathogenic in respect of hemolysis. Koronakis et al [34] showed three types of hemolytic activities (intracellular, cell associated, cell free) in Pv and two types (intracellular, cell associated) in Pm. Here we found that Pp is more hemolytic than Pm that might be the results of three types of hemolytic activities of Pp. Quantization of three hemolytic activities in Pv were measured (Figure 2) where hemolysis caused by cell free supernatant of Proteus was prominent (75%) compared to that of positive control (Triton X-100). However, the hemolysis caused by cell associated and intracellular toxins were 72 and 5.9%, respectively.
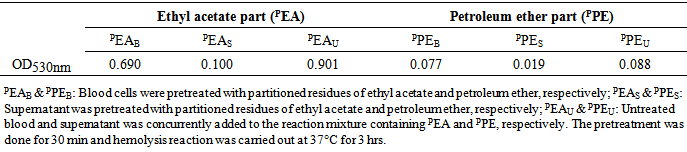
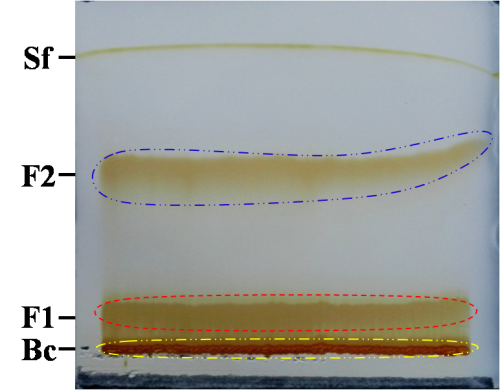 | Figure 5. A representative image of PTLC. Sf: solvent front, F2: separated compound, F1: partially separated compound, Bc: baseline compounds |
|
4. Conclusions
- Hemolysis of human erythrocytes caused by Proteus bacterial toxin was robustly inhibited by alcoholic extract of Tamarindas indica bark. Polyphenols in extract inactivated the toxin and thereby protected blood cells. Further investigation is needed for the isolation and structure elucidation of bioactive polyphenol(s) in Ti-b.
ACKNOWLEDGEMENTS
- Authors thank the Ministry of Science and Technology, Bangladesh for the NST fellowship provided to MR and AW to carry out the research.
Abbreviations
- NHS: Normal human serum, Pm: Proteus mirabilis, Pv: P. vulgaris, Ph: P. hauseri, Pp: P. penneri, CAUTI: Catheter associated UTI, Ti-b: Tamarindus indica bark.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML