-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(4): 123-127
doi:10.5923/j.microbiology.20150504.01
Nasal Carriage Rates of Staphylococcus aureus and CA-Methicillin Resistant Staphylococcus aureus among University Students
Mahde S. Assafi1, Reem Qasim Mohammed1, Nawfal R. Hussein2
1Department of Biology, Faculty of Sciences, University of Zakho, Zakho, Kurdistan region, Iraq
2Department of Internal Medicine, School of Medicine Faculty of Medical Sciences, University of Duhok, Duhok, Kurdistan region, Iraq
Correspondence to: Mahde S. Assafi, Department of Biology, Faculty of Sciences, University of Zakho, Zakho, Kurdistan region, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The treatment of methicillin resistant Staphylococcus aureus (MRSA) infection is challenging because of their high resistance to different antibiotics. The objectives of this study were to determine the prevalence of S.aureus and MRSA nasal carriers among healthy students in Zakho University and to study their resistance pattern to vancomycin. During December 2013 to July 2014, a total of 405 nasal swabs were collected from healthy students and screened for S. aureus. The microorganisms were identified based on morphology and biochemical tests. Then, antibiotic susceptibility to methicillin and vancomycin was determined. The nasal carriage rate of S.aureus and MRSA among participated students were 17.5% (71/405) and 4.2% (17/405), respectively. The highest nasal carriage rate of S.aureus was found in third year students (24.7%, p =0.04). The nasal carriage rate of S.aureus was more common in males than females (20.6% and 14.8%, respectively, p = 0.14). However, the nasal carriage rate of MRSA was more common in females than males (5% and 3% respectively, p = 0.45). All isolates were sensitive to vancomycin.S.aureus and MRSA nasal carriage rates observed amongst university students were comparable to other studies. The transmission of S.aureus and MRSA colonization, infection, and treatment should be explained to the students in order to prevent the spread and control their infections.
Keywords: Nasal carriage, S.aureus, MRSA, Iraq
Cite this paper: Mahde S. Assafi, Reem Qasim Mohammed, Nawfal R. Hussein, Nasal Carriage Rates of Staphylococcus aureus and CA-Methicillin Resistant Staphylococcus aureus among University Students, Journal of Microbiology Research, Vol. 5 No. 4, 2015, pp. 123-127. doi: 10.5923/j.microbiology.20150504.01.
1. Introduction
- Staphylococcus aureus is one of the most successful and adaptable human pathogens and responsible for difficult infections [1]. Overuse and misuse of antibiotics have led to increased levels of antibiotics-resistance. Methicillin was invented for the treatmentof pencillin-resistnat S. aureus [1]. However, methicillin-resistant S. aureus (MRSA) emerged and at the beginning such strains were a challenge in hospitals and health care units. Then, MRSA turned into the public-health problem affecting healthy individuals and has become the most frequent cause of skin and soft-tissue infections in the community [2-4]. As a result and to discriminate between community and healthcare facility strains, community-associated MRSA (CA-MRSA) and health care-associated MRSA (HA-MRSA) have been used. Several studies have examined the prevalence of MRSA nasal carriage among health workers, outpatient settings, injection drug users and medical students [5-8]. Also, researchers have investigated the CA-MRSA carriage rates in a general population [9, 10]. The aims of this paper were to study the prevalence of S. aureus and MRSA in the anterior nares of healthy students and to study their susceptibility to vancomycin at Zakho university, Kurdistan region, Iraq.
2. Materials and Methods
- Setting and sample collectionA cross-sectional study was conducted in University of Zakho, Zakho city, Kurdistan region-Iraq. The study was conducted with the approval of ethics committee in the University of Zakho. A total of 432 students aged 19 to 25 years were participated in this study from December 2013 to July 2014. Nasal swabs (moistened with sterile distilled water) were taken from anterior nares of the participants. The swab was inserted about 2 cm into the naris and directly transported for specimen processing.Laboratory analysis of S. aureus and MRSA isolates Nasal swabs were screened for S. aureus and MRSA. Samples were directly cultured on Mannitol Salt Agar (Oxoid) and incubated at 35ºC for 24 hours. Positive colonies on mannitol salt agar were identified as S. aureus strains based on morphology, Gram stain and biochemical tests including catalase and coagulase.The bacterial suspension was adjusted to the concentration of 0.5 McFarland and then 10μl inoculum was spread on the agar plate (final concentration = 106 CFU/ml). Antimicrobial susceptibility testing to oxacillin was carried out according to Clinical Laboratory Standards Institute (CLSI) recommendations using Kirby-Bauer disk diffusion and agar dilution assay methods using Muller-Hinton agar (Oxoid Limited, Hampshire, England). BHI agar plates supplemented with 6 μg/ml vancomycin were used for testing of strains for vancomycin resistance [11].Data analysisStatistical analysis of data was performed by using the chi-squared test with significance set at a p value of <0.05 using Minitab 15 software (Minitab Ltd., Coventry, UK).
3. Results
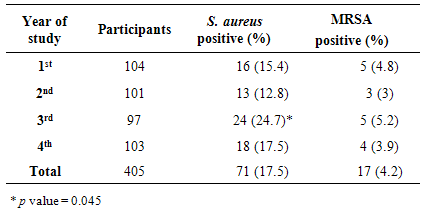
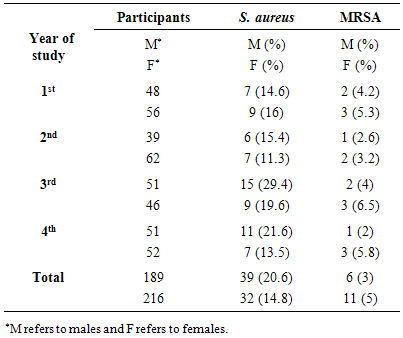
- Because hospitalization and admission to healthcare facilities increase the risk of MRSA colonization, we excluded every student with history of hospitalization, surgery, dialysis or residence in a long-term care facility within one year of the MRSA culture date, the presence of an indwelling catheter or a percutaneous device at the time of culture and history of previous isolation of MRSA. A total of 432 participants volunteered in this study, 27 students (6.25%) were excluded because of the above mentioned factors. The included students (405) were examined for presence of S. aureus and MRSA. The male and female participants were 47% (189/405) and 53% (216/405) respectively. A total of 71 S. aureus and 17 MRSA were isolated from 405 participants, giving an overall positivity rate of 17.5% and 4.2%, respectively (Table 1). With regard to the year of study, prevalence of S. aureus rate was higher in the 3rd stage students 24.7% (24/97) than students in other stages (p=0.045) (Table 1).
|
|
4. Discussion
- S. aureus is a normal commensal in the nose of about 25-30% of healthy people [12]. Different factors contribute to the transmission of this microorganism such as crowded living conditions, and poor hygiene [13, 14]. The presence of S. aureus on the skin appears to play a key role in the pathogenesis of infection with S. aureus [15, 16]. Eradication of S. aureus from the nose reduced the incidence of invasive infection [16, 17]. In this study, the prevalence of S. aureus nasal carriage among students was 17.5%. This result is comparable with other studies from Iraq, Iran and Turkey [9, 18-20] and is lower than those reported in Nigeria, Ethiopia and India [21-25]. S. aureus nasal carriage rate was significantly higher among third year students. Outbreaks have been reported among different groups such as athletes, military recruits, prisons detainees, livestock handlers, pet owners, intravenous drug users, students [14, 26, 27]. There are different factors contributing to spread of S. aureus and MRSA including crowded living conditions, poor hygiene habits, close skin-to-skin contact, sharing of personal items, frequent antibiotic exposure, hospitalization and intravenous drug abuse [28]. It was found that the carriage rate of S. aureus was higher on participants who had been hospitalized within the past 1 year than those who had not [7]. One factor that could assist to distribute S. aureus among third year students is that the colonization of these bacteria among household members is shown to be higher than rates reported among the general population [4]. Member of this group of students may live in shared houses or accommodate internal departments of the university. More study is needed to explore this result. MRSA was identified as a nosocomial pathogen which is one of the causative agents of healthcare associated infections worldwide. MRSA has traditionally considered as associated with healthcare settings. However, new strains have emerged in the community and an increasing numbers were observed in people who have not been hospitalized or had a medical procedure [29, 30]. Different studies showed variable rate (0.8 - 36%) of MRSA nasal colonization [7, 31-34]. In our region, the nasal carriage rate of MRSA was 4.2%. Combination of factors could contribute to the nasal carriage of S. aureus and MRSA among population including host, geographical, environmental and bacterial factors. Epidemiological evidence supports that the mechanism for transmission S. aureus and MRSA usually via direct contact with patients and other close contacts [35]. In our study, male students had a highest prevalence of S. aureus nasal carriage. This is in agreement with other studies that found that S. aureus nasal carriage rate is higher in males than females [7, 36, 37]. In agreement with other study, it was found that the prevalence of MRSA was more common in females than males [38, 39]. A similar result was abstained by Braga et al. [28] who showed that the prevalence of S. aureus are more common in males and MRSA are more prevalence in females. It is observed that females harbour a greater diversity of bacteria on their hands than males, but it is not obvious whether this is due to physiological factors or differences in hygiene and cosmetic usage [40]. Furthermore, the microbial differences between male and female could be due to the physiological and anatomical differences between genders cutaneous environments such as sweat, sebum and hormone production [41]. Vancomycin is considered one of the last options of treatment for S. aureus infections that are resistant to other antibiotics. Analysis of different studies revealed the emergence of Vancomycin-Resistant Staphylococcus aureus (VRSA) from different parts of the neighboring countries [42-45]. Fortunately, no vancomycin-resistant S. aureus (VRSA) isolates was found in this study. In conclusion, the nasal carriage rate of S. aureus and MRSA observed in this study was relatively low and comparable to other studies in surrounding area. No vancomycin-resistant S. aureus was observed in our study. The implications of S. aureus and MRSA colonization, infection, and treatment should be explained to the students in order to prevent the spread and control their infections.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML