-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(3): 95-100
doi:10.5923/j.microbiology.20150503.03
Studies on the Antifungal Activity of Lactobacillus plantarum and Lactobacillus fermentum on Spoilage Fungi of Tomato Fruit
Fowoyo Patience Temitope, Uzoma Eberechukwu Oluchi
Biosciences Department, Salem University, Lokoja, Nigeria
Correspondence to: Fowoyo Patience Temitope, Biosciences Department, Salem University, Lokoja, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
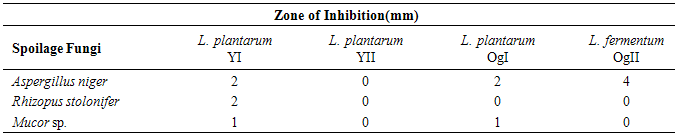
Tomato fruit undergoes rapid deterioration due to microbial spoilage caused mainly by fungi. The aim of this work is to determine the inhibitory effect of Lactobacillus plantarum and Lactobacillus fermentum against spoilage fungi of tomato fruit Lactobacillus plantarum and Lactobacillus fermentum were isolated from five different yoghurt samples and three different ogi samples by plating on MRS agar plates. Spoilage fungi of tomato were isolated on PDA and were identified as Aspergillus niger, Rhizopus stolonifer and Mucor sp. using their colonial morphology and microscopic characteristics. The antifungal activity of Lactobacillus plantarum and Lactobacillus fermentum against the isolated spoilage fungi of tomato was determined using the agar well diffusion technique. The result showed that after 24h of incubation, Aspergillus niger was inhibited by Lactobacillus plantarum with zone of inhibition of 2mm while Lactobacillus fermentumshowed a higher inhibition of 4mm.Rhizopusstoloniferwas only inhibited by Lactobacillus plantarum with a zone of inhibition of 2mm and Mucorsp.was also inhibited by Lactobacillus plantarumwith zone of inhibition of 1mm.The antifungal activity exhibited by Lactobacillus plantarum and Lactobacillus fermentum showed that they are highly effective against eliminating spoilage fungi of tomato. However, further studies should be conducted to determine the metabolite responsible for the antifungal activity and methods designed so that they can be used as aerosols or used in modified atmosphere packaging of tomato so as to increase the shelf life of tomatoes.
Keywords: Antifungal, Lactic Acid bacteria, Spoilage fungi, Tomato fruit
Cite this paper: Fowoyo Patience Temitope, Uzoma Eberechukwu Oluchi, Studies on the Antifungal Activity of Lactobacillus plantarum and Lactobacillus fermentum on Spoilage Fungi of Tomato Fruit, Journal of Microbiology Research, Vol. 5 No. 3, 2015, pp. 95-100. doi: 10.5923/j.microbiology.20150503.03.
Article Outline
1. Introduction
- Tomato is a fleshy fruit and it is the world’s largest vegetable crop after potato. Tomatoes are eaten directly either as raw vegetable or consumed in a variety of processed products like ketch-up, sauce, chutney, juice, diced, soup, paste, puree etc. It is a rich source of vitamin A and C, and also contains minerals like iron, phosphorus [8].Tomatoes are highly susceptible to fruit decays, from the field through postharvest handling. Postharvest decays result in wounds, bruised tissue and fruit softening [12]. The spoilage of fresh fruit and vegetable is generally due to physiological disorder and microbial spoilage caused by fungi and bacteria. Microbial spoilage of fruits and vegetables is known as rot, which consist of colour changes, loss of texture and often off odour. Fungal pathogens cause devastating losses of crops and postharvest fruits throughout the world [23]. Organisms that cause food spoilage - molds, yeasts and bacteria are always present in the air, water and soil. Fungi are the most important and prevalent pathogens, infecting a wide range of fruit and causing destructive and economically important losses of fruits during storage, transportation and marketing [20]. Different fungal species such as Alternaria, Fusarium, Penicillium, Mucor, Rhizopus, Aspergillus, Geotrichum, Phytophthora and Botrytis have been implicated as spoilage organisms [20], Aspergillus niger, Rhizopus stolonifer and Mucor sp. have being isolated from spoilt tomatoes [14]. Aspergillus niger been reported to cause a disease called black mold on certain fruits and vegetables such as grapes, tomato, onions and peanuts [18].Rhizopus stolonifer is a fungus known to be the causal organism of soft rot of fruits and vegetables [3]. Mucor sp. are primarily post-harvest rots but also occur on ripe fruit in the field. Infected fruit are initially slightly discoloured, gradually turning pale brown. The tissue rapidly softens and collapses and, under humid conditions, the fruit is soon covered with a dense, fluffy white mycelium which bears stiff, black-headed sporangia. Other common diseases of tomato caused by fungi are septoria leaf spot caused by the fungus Septoria lycopersici, early blight caused by the fungus Alternaria solani, late blight, caused by the fungus Phytophthora infestans [12]. Fusarium and Verticillium wilts are also quite common [19]. Lactic acid bacteria have well been established as organisms that produce inhibitory compounds against bacteria and fungi. The antimicrobial activity of lactic acid bacteria is primarily attributed to a wide variety of active antagonistic metabolites that includes; organic acid acids (lactic, acetic, formic, propionic, butyric, hydroxyl- phenyllactic acid, and phenyllactic acid), or antagonistic compounds (carbon dioxide, ethanol, hydro peroxide, fatty acids,acetoin, diacetyl), bacteriocins (nisin, reuterin, reutericyclin, pediocin, lacticin, enterocin, etc.) or bacteriocin-like inhibitory substances ([13], [15], [17]). The production of these metabolites counteracts the growth of spoilage organisms present in tomatoes ([17]). The aim of this work is to determine the antifungal activity of Lactobacillus fermentum and Lactobacillus plantarum against spoilage fungi of tomatoes.
2. Materials and Methods
2.1. Sample Collection (Yoghurt, Ogi and Spoilt Tomatoes)
- Five different local brands of yoghurt were purchased from four vendors from different locations (Ganaja junction, Nataco, Salem University cafe and Old Market in Lokoja, Kogi State Nigeria). Three samples of Ogi were purchased from three different hawkers from New Market in Lokoja, Kogi State Nigeria. Six samples of spoilt tomatoes were purchased from different vendors from two major markets (the Old and New Market in Lokoja, Kogi State, Nigeria). All the samples were transported aseptically to the laboratory in separate sterile bags where analysis was carried out.
2.2. Isolation of Lactic Acid Bacteria from Ogi and Yoghurt
- The method used by [22] was employed. Yoghurt and Ogi samples were serially diluted to 10-5 using sterile peptone water. One ml of the aliquot suspension of the 10-5 dilutions were inoculated into sterile Petri dishes and MRS Agar (de Man Rogosa Sharpe agar) was poured into the plates and mixed gently. The plates were allowed to solidify and incubated at 37°C for 48h under anaerobic condition using a gaspak jar.
2.3. Isolation of Fungi from Spoilt Tomato fruit
- The method described by [5] was employed. A sterile scalpel was used to cut an infected portion of a spoilt tomato fruit. The cut, infected portion was used to inoculate prepared Potato Dextrose Agar plate. The inoculated plates were incubated at 25°C for 3-5 days and were observed until the organisms became fully grown and covered the agar plates.
2.4. Isolation of Pure Cultures of Lactic Acid Bacteria and Fungi
- After incubation, distinct colonies were sub-cultured on MRS agar plates and repeatedly streaked on MRS agar plates and were incubated to obtain a pure culture. Also growth on PDA plates were repeatedly sub-cultured to obtain pure cultures. Slants were prepared respectively in McCartney bottles, inoculated and incubated at 37°C for 24h (Bacteria) and 25°C for 3-5days (Fungi). Pure cultures were stored in the refrigerator at a temperature of 4°C as stock culture.
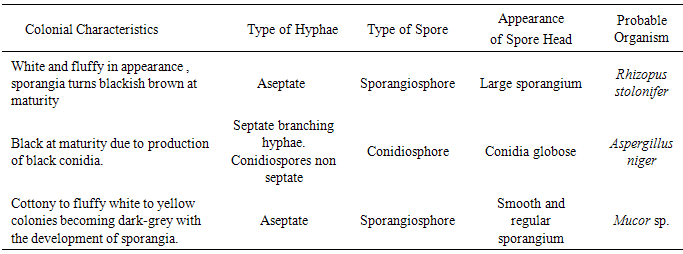
2.5. Identification of Fungal Isolates
- Fungal isolates were identified using both colonial morphology [6] and microscopic characteristics [4]. The colonial morphology were determined by observation of visual appearance involving colour and mycelia formation while for the microscopic characteristics; an inoculating needle was used to pick a mycelia strand from the fungal plates and placed onto the slide and stained with 1-2 drops of lactophenol blue and covered with a cover slip and examined under the light microscope. Identification was based on the type of mycelia structure and spore formation.
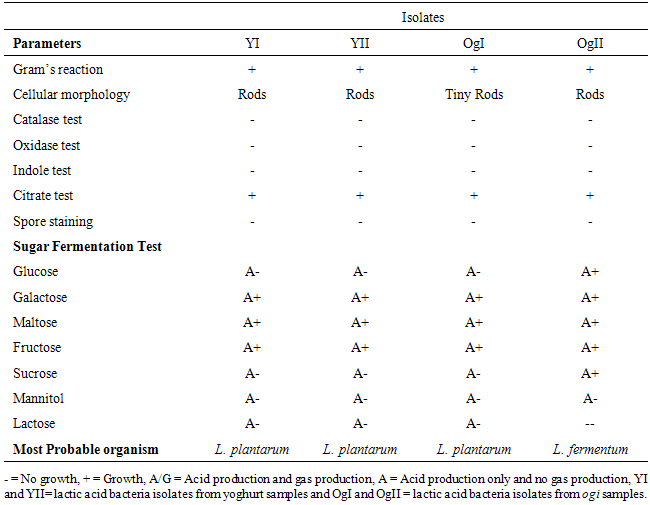
2.6. Identification of Lactic Acid Bacteria Isolates
- The lactic acid bacteria isolates were identified using colonial morphology and various biochemical tests including gram staining, spore staining, catalase test, citrate utilization test, indole test, motility test, Oxidase test and sugar fermentation test.
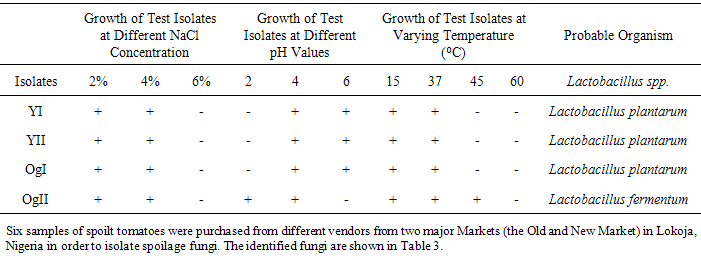
2.7. Determination of the Effect of pH on the Growth of Lactic Acid Bacteria Isolates
- The method described by [21] was employed. The lactic acid bacteria isolates were inoculated into sterile MRS broth tubes with varying pH values (pH 2, 4, and 6) using 2M acetic acid and incubated at 37°C for 24h. Turbidity in the broth indicated growth.
2.8. Determination of the Effect of NaCl Concentration on the Growth of Lactic Acid Bacteria Isolates
- The method described by [2], [7] was employed. Salt tolerance of selected bacterial cultures were assessed at varying concentration of 2%, 4%, 6% NaCl in MRS broth and incubated at 37°C for 24h. Growth was determined by change in turbidity of the culture medium.
2.9. Determination of the Effect of Temperature on the Growth of Lactic Acid Bacterial Isolates
- The method described by [21] was used. The lactic acid bacteria isolates were inoculated into sterile MRS broth and incubated at varying temperatures of 15, 37, 45 and 60°C at 37°C for 24h. Growth was determined by change in turbidity of the culture medium.Determination of Antifungal Activity of L. Plantarum and L. fermentum Metabolites against Rhizopus stolonifer, Aspergillus niger and Mucor sp. from Spoilt Tomato Fruit.The agar well diffusion method described by [14] was employed. Fungal isolates (Rhizopus stolonifer, Aspergillus niger and Mucor sp.) obtained from spoilt tomatoes were aseptically sub-cultured onto sterile potato dextrose agar (PDA) plate. The PDA plates were incubated at the temperature of 25°C for 3-5d. Test isolates of L. plantarum and L. fermentum were grown in MRS broth for 18-24h. The broth cultures of L. plantarum and L. fermentum were centrifuged at 10,000 rpm for 30 minutes. The supernatant containing the metabolites were obtained and 100µl of the supernatant was transferred into wells (6mm diameter) bored in PDA plate previously seeded with the spoilage fungi spores obtained from spoilt tomatoes. The culture plates were incubated at 25°C for 7 days and observed for zones of inhibition.
3. Results
- Five different local brands of yoghurt from Ganaja junction, Nataco, Salem University Cafe and Old Market in Lokoja, Nigeria and three samples of Ogi from New Market in Lokoja, Nigeria were analysed microbiologically for the incidence of L. plantarum and L. fermentum as shown in Table 1.
|
|
|
|
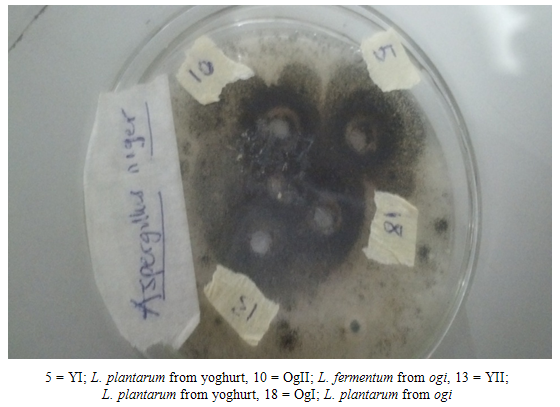 | Plate 1. Antimicrobial Activity of Lactobacillus plantarum and Lactobacillus fermentum against Aspergillus niger |
4. Discussion
- Lactobacillus plantarum and Lactobacillus fermentum were isolated from yoghurt and ogi samples and this conforms to the work by [9], [16] which show the incidence of these organisms in the food samples.Lactobacillus plantarum and Lactobacillus fermentum are both Gram positive rods and share similar attributes however, they become easily distinguishable by comparing their physiological attributes such as tolerance to NaCl, pH and varying temperatures and this is in accordance with the work of [21]. Aspergillus niger, Rhizopus stolonifer and Mucor sp. were isolated from spoilt tomatoes which indicated that they were responsible for spoilage and this conforms to the work of [14]. Lactobacillus plantarum and Lactobacillus fermentum has being studied to exhibit antimicrobial activity against spoilage fungi as shown in the work of [1]. [10] suggested that the antifungal activity of Lactobacillus plantarum could be the result of many organic acids such as lactic acid, acetic acid, hydrogen peroxide, lactoperoxidase system with H2O2, lysozyme, reuterin, diacetyl, fatty acids, bacteriocins, lactic, and phenyllactic acids. Since more than one compound is responsible for the antimicrobial activity, the specific compound or combination of compounds need to be further studied for their potential use as food bio-preservatives. The use of bio-preservatives that can extend the shelf life of tomatoes will go a long way to solve the problem of waste and as well extend the keeping time in homes. The result obtained from this work revealed that the antifungal activity of Lactobacillus plantarum and Lactobacillus fermentum strains showed significant effect against spoilage fungi.However, further studies should be conducted to elucidate the nature of the antagonistic metabolites produced by some strains of lactic acid bacteria and also to establish its technological implementation on a larger scale in the agricultural sector to minimize the high rate of food spoilage in our society and to save our economy from agricultural losses.It is therefore recommended that the metabolites of Lactobacillus plantarum and Lactobacillus fermentum can be used as bioaerosols or agricultural sprays during post harvesting. They can also be used in modified atmosphere packaging of tomato so as to increase the shelf life tomatoes. This can be done by the application of these metabolites produced by lactic acid bacteria in packaging materials during post-harvest and distribution as this will implementation on a larger scale in the agricultural sector to minimize the high rate of food spoilage in our society and to save our economy from agricultural losses.This can be done by the application of these metabolites produced by lactic acid bacteria in packaging materials during post- harvest and distribution as this will help to inhibit the growth of spoilage organism thereby ensuring food safety.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML