-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(3): 84-94
doi:10.5923/j.microbiology.20150503.02
Effect of Selected Lactic Acid Bacteria on Growth of Aspergillus flavus and Aflatoxin B1 Production in Kutukutu
Tchikoua Roger1, Tatsadjieu Ngouné Léopold2, Mbofung Carl M. F.1
1Nationnal School of Agro-Industrial Sciences, Ngaoundere, Cameroon
2University Institute of Technology, University of Ngaoundere, Cameroon
Correspondence to: Tatsadjieu Ngouné Léopold, University Institute of Technology, University of Ngaoundere, Cameroon.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Inhibition of Aspergillusflavusgrowth and degradation of aflatoxin B1 (AFB1) by six Lactic Acid Bacteria (LAB) (Lactobacillus brevis G11, Lactobacillus brevis G25, Lactobacillusbuchneri M11, LactobacilluscellobiosusM41, LactobacillusfermentumN33 and Lactobacillusfermentum N25) were studied in Kutukutu, a fermented maize-based dough largely consumed in the Northern part of Cameroon. A total of twenty nine samples of Kutukutu were obtained from the markets in Garoua, Maroua and Ngaoundere (three towns of the Northern part of Cameroon). Then the occurrence of AFB1 was determined using Enzyme Linked Immuno-Sorbent Assay (ELISA). Afterward, the Kutukutu was prepared in the Laboratory conditions following the traditional method. This Kutukutu was divided into several batches. The first six batches were inoculated with the spores of Aspergillusflavus M15 and different LAB. The second six batches were inoculated with AFB1 along with LAB. The batches were then incubated at 25°C and 37°C for the first and second batch respectively for 120 hours. At 24, 48, 72, 96 and 120 hours, mycelia growth and AFB1 were monitored in different Kutukutu. The results showed that all the Kutukutu samples obtained in the markets of Garoua, Maroua and Ngaoundere were contaminated by AFB1 and those of Maroua contained the highest concentration of AFB1 (2.3 ppb). After 24 hours of incubation, the growth of Aspergillusflavus M15 was totally inhibited in the presence of L. brevis G11, L. buchneri M11 and L. cellobiosus M41. The similar performance was observed after 120 hours with L. fermentum N25 and L. fermentum N33. The incubation of Kutukutucontaminated with aflatoxin showed after 120 hours that the AFB1 was degraded by the LAB in the following order L. buchneri M11 (64.2%) >L. brevis G25 (63%) > L. fermentumN33 (57.2) >L. cellobiosus M41 (52.3%) >L. fermentumN25 (45.3%) >L. brevis G11 (43.9%).The present findings highlight the possibility of exploiting the LAB potential in the control of aflatoxinogenic strains of A.flavusin Kutukutu.
Keywords: Aflatoxin B1, Aspergillusflavus M15, Lactic Acid Bacteria, Fermentation
Cite this paper: Tchikoua Roger, Tatsadjieu Ngouné Léopold, Mbofung Carl M. F., Effect of Selected Lactic Acid Bacteria on Growth of Aspergillus flavus and Aflatoxin B1 Production in Kutukutu, Journal of Microbiology Research, Vol. 5 No. 3, 2015, pp. 84-94. doi: 10.5923/j.microbiology.20150503.02.
Article Outline
1. Introduction
- Kutukutu is a popular traditional fermented paste obtained from maize (Zea mays). It is usually consumed in Northern part of Cameroon by adults (as breakfast) and young children (as complementary food). During the Ramadan period, Kutukutu is frequently taken by the Muslim community before consumption of any other food [1]. Traditional process of Kutukutu’s production involves several steps such as: sorting: washing (optional); soaking the maize grains; grinding; filtering the paste and decanting [1]. After the production, Kutukutu is commonly conserved at ambient temperature in water. The renewal of this water takes place as soon as the signs of deterioration (fermentation) are perceptible. One of the most serious problems to confront the quality of Kutukutu is the presence of mycotoxins which are produced by different species of the genus Aspergillus or Penicillium. Aflatoxinsare mycotoxins of greatest public health concern. It is highly toxic, mutagenic, teratogenic and carcinogenic. It is also a causative agent in human hepatic and extra-hepatic carcinogenesis [2-4]. In Africa, there are ample evidences of the direct and negative effects of aflatoxin on human health through the increase of incidence of liver cancer and its potential synergistic effect on hepatitis B [5]. Moreover, recent studies have pointed out the immunosuppressive properties of aflatoxin [4-6]. A study conducted in Togo (West Africa) revealed a high frequency of kwashiorkor due to the presence of aflatoxin in the blood of 99% of the children who consumed porridge prepared with contaminated cereal. In view of health issues and economic considerations related to the presence of aflatoxin or aflatoxigenic strains in foods, the search for antifungal agents is still of today’s interest [7, 8].Measures have been taken to reduce the level of aflatoxin in food and prevent fungal growth in stored grains. Addition of chemicals such as benzoic acid and sorbic acid are preventive methods used so far. However, the consequences such as resistance of the species, the residual level in food, remain the major problems of their use [9]. Moreover, the consumers are looking and demanding for safe products without chemical preservatives and good shelf life. Due to these strict expectations of consumers, the concept of biopreservation is gaining popularity. This concept refers to extension of shelf life and enhanced safety of foods by the growth of the natural or added microflora and their antimicrobial products [10].Among the microorganisms which can be potentially used as biopreservatives, LAB has demonstrated their efficacy with their Generally Recognized As Safe (GRAS) status, they have traditionally been used in food and animal feed, sauerkraut and silage. The mechanism behind the inhibition of fungi growth hand the preserving effects of LAB relate mainly to the formation of organic acids, hydrogen peroxide, competition for nutrients and production of antimicrobial substances [11, 12, 13]. However, there is reported strain variation that boosts scientists to a perpetual exploration of strains with better potential.In view of the high level of contamination of maize sampled in north Cameroon and the awful consequences on the health of consumers, the present investigation aim to improve the safety of fermented Kutukutu by developing a suitable biological detoxification procedure using selected LAB strains, which may be adopted for traditional process of fermentation.
2. Materials and Methods
2.1. Concentration of Aflatoxin B1 in Kutukutu sold in North Cameroon
2.1.1. Samples Collection
- Twenty nine samples of Kutukutu, of 100g each, were collected at Maroua (9 samples), Ngaoundere (11 samples) and Garoua (9 samples) during the dry season in January 2013. The samples of Kutukutu were aseptically transferred to storage bags, maintained in ice and transported immediately to the laboratory for further analyses.
2.1.2. Determination of Aflatoxin B1 in Kutukutu soldin North Cameroon
- Kutukutu collected were first dried to constant weight at 60°C and AFB1 was extracted using the method described by [14]. Quantitative estimation of AFB1 was made using Enzyme Linked Immuno Sorbent Assay (ELISA). Immuno enzymatic kits (Celer AFB1, Techna, Italy) were used. The Absorbance of samples was measured at 450nm using a plate reader (Metertech, 6+ Model, Version 2.01).
2.2. Preparation and Treatment of Kutukutu
2.2.1 Maize sample
- For the preparation of Kutukutu, freshly harvested maize grains of CMS 8501 variety obtained from the Institute of Research and Development, Ngaoundere-Cameroon were used in the present investigation. No infestation was detected in the grains.
2.2.2. Fungal Strain and Production of Conidia
- A. flavusM15, an aflatoxigenic strain, obtained from the Microbiology Laboratory of the National School of Agro-Industrial Sciences (University of Ngaoundere, Cameroon), was used as test microorganism. It was grown on Sabouraud dextrose agar (Difco, Detroit, MI) at 25°C for 6 days. Conidia were collected after vigorous shaking of slants with sterile peptone water (0.2% w/v). Mycelial debris were removed from conidia suspension by filtering twice through several layers of sterile damp cheese cloth [15]. The concentration of conidia was determined with a haemocytometer. The suspension was stored at 4°C until use.
2.2.3. Lactic Acid Bacteria Strains
- Six LAB isolates (L. brevis G11, L. brevis G25, L. buchneri M11, L. cellobiosus M41, L. fermentum N33 and L. fermentum N25) were obtained from the Microbiology Laboratory of the National School of Agro-Industrial Sciences (University of Ngaoundere, Cameroon). Strains were subcultured from a stock culture in de Man Rogosa Sharpe broth (MRS, Difco) and incubated at 30°C for 72 hours. The perfectly insulated colonies were inoculated in test tubes containing 10mL of MRS broth and incubated at 30°C for 16 hours. The resulting preparation was centrifuged at 3000 rpm/min for 10 min and the resulting pellet was washed in 10mL of physiological peptone water (peptone 1g in saline solution (0.85% NaCl), pH 7.2) and centrifuged again at 3000 rpm/10mn. The pellet obtained was suspended in 10mL saline water. The concentration of viable cells was adjusted at 109 CFU/mL using McFarland Standard tube No.4.
2.2.4. Preparation of Kutukutu
- Kutukutu was produced in laboratory following the process as illustrated in figure 1. After sorting, the maize grains were washed and steeped in sterile distilled water containing benzoic acid 6% (w/v) (E210) for 24 hours at room temperature. The grains were then steeped again in sterile distilled water for 48 hours at room temperature and grinded using a plate disc mill. The paste obtained was mixed (1/3 w/v) with sterile distilled water and sieved through a sieve of mesh 200µm. The paste was collected in a sterile container after decantation for 24 hours at room temperature.
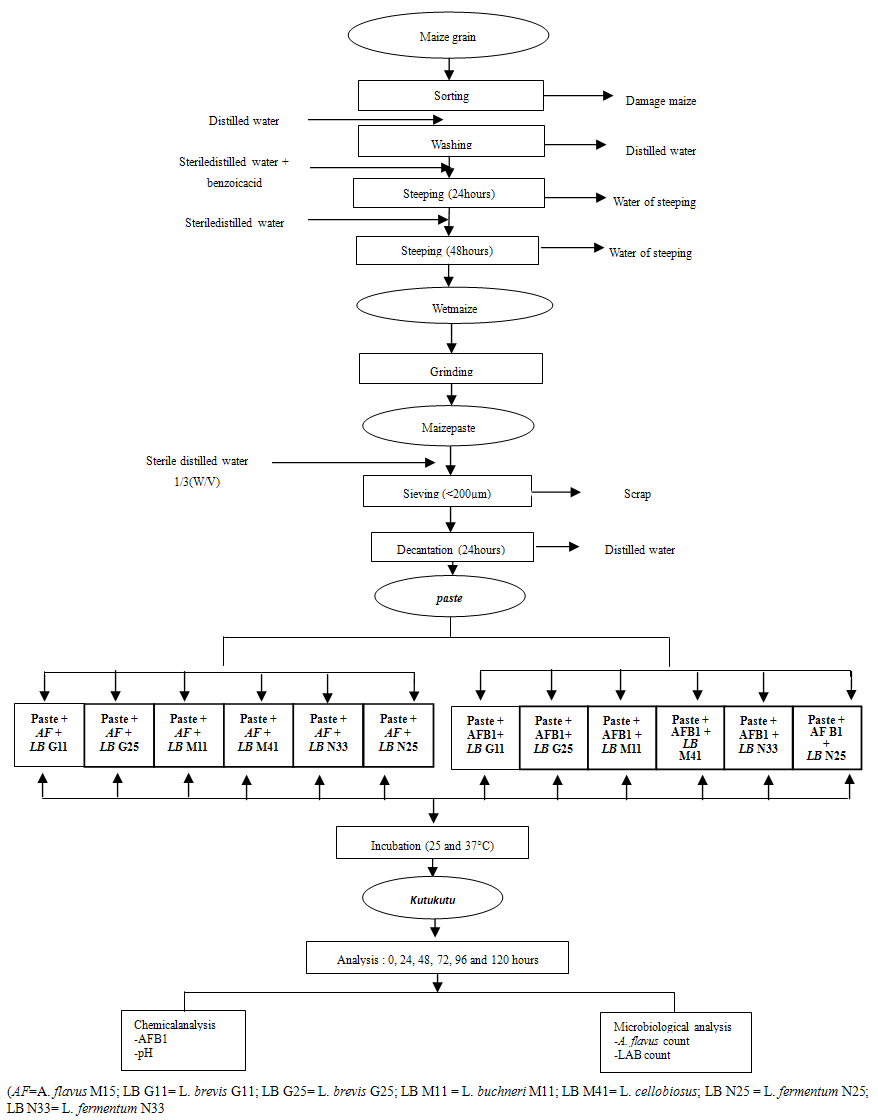 | Figure 1. Diagram of inoculation of Kutukutu with LAB, A. flavus M 15 and AFB1 |
2.2.5. Inoculation of kutukutu
- To assess the influence of LAB on fungal growth in Kutukutu, 700g of paste was inoculated with spores of A. flavus M15 and LAB strain at the concentrations 106 spores/mL and 109 CFU/mL, respectively (figure 1). The preparations were covered and incubated at 25°C for 120 hours. Aliquots were collected every 24 hours for microbiological analyses.To evaluate the capacity of LAB to reduce AFB1in Kutukutu, 300μg of paste was mixed with a pure solution of AFB1(40 ppb) and 450μl of LAB inoculum (109 CFU/mL) was added, mixed and incubated at 37°C for 120 hours (Figure 1). Aliquots were collected every 24 hours for microbiological analyses.
2.3. PH Determination
- The pH of the Kutukutu was determined using a pH-meter (Consort C863, Belgique) according to the method of [16].
2.4. Inhibition of Fungal Growth in Kutukutu
- The mould count was carried out by the method described by [17]. One gram of Kutukutu was introduced in 9mL of saline water (0.85% NaCl) to provide the first dilution (10-1). Serial dilutions were then performed and 0.1mL of the stock solution and all dilutions were inoculated on PDA medium. The preparations were incubated at 25°C for 48 hours.
2.5. Growth of Lactic Acid Bacteria
- During fermentation of Kutukutu, LAB in the presence of A. flavusM15was enumerated using the method of [18]. For the analysis, 25g of Kutukutu were crushed and introduced into a conical flask containing a volume of 225ml of saline (0.85% NaCl) to provide a first dilution of 10-1. Serial dilutions were performed by introducing 1ml of the mixture in 9ml of saline contained in a sterile test tube. Then, 0.1ml of the stock solution and all dilutions were placed on MRS agar and incubated at 30°C in an anaerobic jar. The enumeration of LAB was done after 48 hours of incubation in Petri dishes containing between 30 and 300 colonies.
2.6. Aflatoxin B1 Reduction
- AFB1 reduction in Kutukutu was performed according to the method described by [19]. Quantitative reduction of AFB1 was determined by using an ELISA KIT every 24 hours during 120 hours of fermentation.
2.7. Statistical Analysis
- The results were analyzed using Statgraphics 5.0 (1998) for the analysis of variance (ANOVA), calculation of averages and standard deviations. Sigma plot 11.0 software was used to draw the curves.
3. Results and Discussion
3.1. Content of Aflatoxin B1 in Kutukutu
- The AFB1 content in Kutukutu sold in Maroua, Ngaoundere and Garoua after a spontaneous fermentation is shown in Figure 2. These results showed that, the Kutukutu sold in these 03 towns were contaminated with AFB1. But, the levels of AFB1 in Kutukutu vary according to the locality. The Kutukutu of Maroua registered the highest rate of AFB1 (2.3±0.5 ppb) followed by the town of Ngaoundere (1.3±0.4 ppb). The rate of AFB1 in the Kutukutu of Maroua was higher than the standard norm fixed by the European Union. The maximum permissible limits for AFB1 should not exceed 2 ppb in peanuts, nuts, dried fruit, processed products as well as cereals and their derivatives [20].
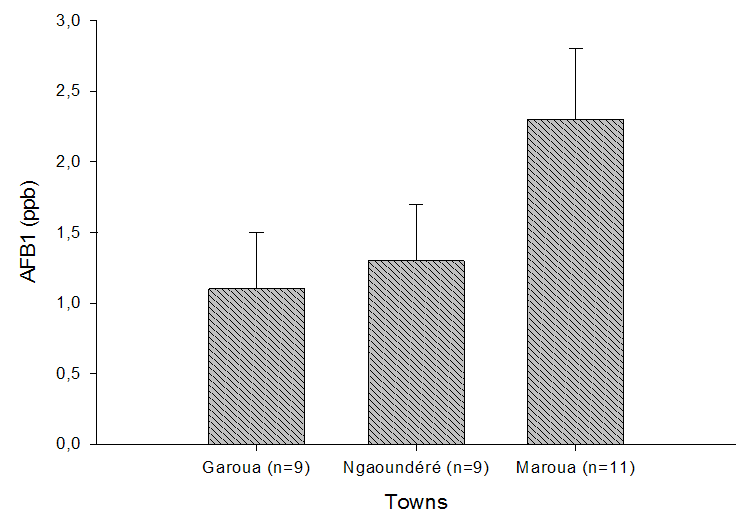 | Figure 2. AFB1 content in Kutukutu sold in Maroua, Garoua and Ngaoundere |
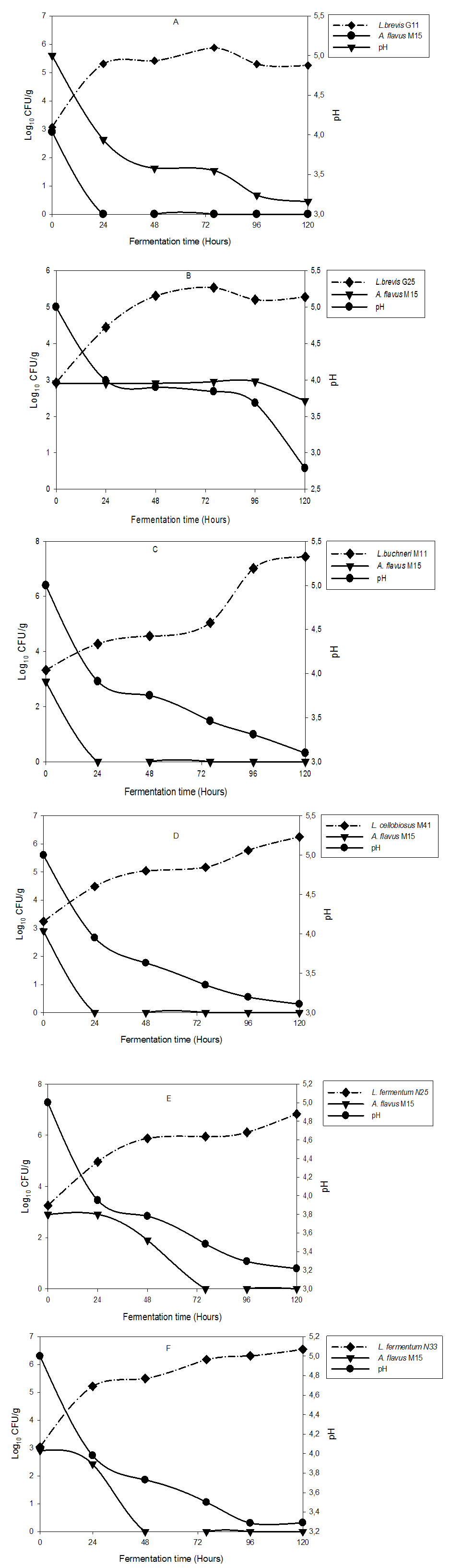
3.2. PH Determination
- The pH change in Kutukutu fermented with LAB is shown in Figure 3. After 120 hours of fermentation, the Kutukutu inoculated with L. brevis G25 had the lowest pH (2.7) follow by pH (3.1) of Kutukutu inoculated with L. buchneri M11 and L. cellobiosus M41.
3.3. Inhibition of Aspergillus flavus M15 growth
- The effect of fermentation of Kutukutu by various LAB on A. flavus M15 growth is shown in figure 3. A. flavus M15 was completely inhibited after 24 hours of fermentation of Kutukutu by L. buchneri M11, L. brevis G11 and L. cellobiosusM41, while control tube exhibited an increase in the number of spores during fermentation. After 48 hours of fermentation, the growth of A. flavusM15 was reduced to 90% in the Kutukutu fermented with L. fermentum N25. At the same time, the growth of A. flavus M15 was completely inhibited by L. fermentum N33. The antifungal properties of LAB have already been reported by a few authors. Most of them belong to the genera Lactococcus and Lactobacillus. Zuo et al. [25] showed the antifungal activity of L. caseiCGMCC1 against A. flavus in liquid medium. Omemu [26] showed that the growth of fungal populationwas significantly (P<0.05) reduced from 6.8 to 3.7 Log10CFU/g after 12 hours of fermentation of Ogi (fermented corn paste). Similarly, Roy et al. [27] showed antifungal activity of six LAB against A. flavus IARI. The interest for LAB in biopreservation of food is growing. Their efficacy lays on the production of wide range of antifungal and antimicrobial compounds such as lactic acid, acetic acid, and bacteriocin. Lavermicocca et al. [28] demonstrated that the antifungal compounds such as phenyllactic acid and 4-hydrophenyllactic acid were produced by Lactobacillus plantarum.
3.4. Reduction of Aflatoxin B1 in Kutukutu
- The figure 4 (A and B) give the removal percentage of AFB1 in Kutukutu fermented with LAB. It was observed soon after 24 hours of incubation, a reduction of AFB1 in Kutukutu fermented with all the LAB. A significant decrease of AFB1 was observed in Kutukutu fermented with L. fermentum N33 (21.8%) and L. brevis G25 (19.1%). However, the highest AFB1 reduction ranging from 6.5 to 2.3 ppb (64.2%) and 6.5 to 2.4 ppb (63%) was observed respectively in Kutukutu fermented with L. buchneri M11 and L. brevis G25 after 120 hours. Studies conducted in fermented foods also showed the reduced of AFB1 by LAB [29].
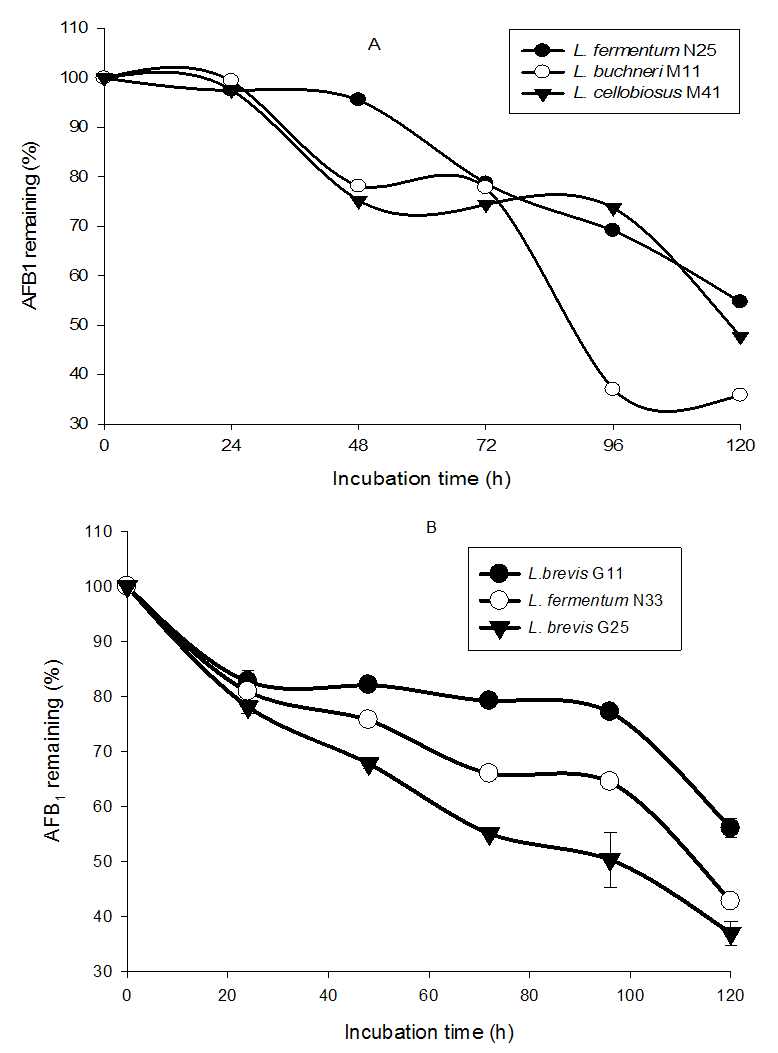 | Figure 4. Remaining percentage of AFB1 in Kutukutu fermented with LAB (A = L. fermentum N25, L. cellobiosus M41 and L. buchneri M11; B = L. brevis G11, L. fermentumN33 and L. brevis G25) |
3.5. Growth of Lactic Acid Bacteria
- The growth of LAB in Kutukutu in the presence of A. flavusM15 is presented in figure 3. The results show that, even in the presence of the moulds, we observed the growth of LAB during the fermentation. This increase of LAB was ranging from 2.9 to 7.4 Log10CFU/g. Conversely, the moulds were inhibited in the presence of low pH it was observed that the LAB can multiply with weak pH (2.7-3.1) during fermentation (Figure 3). This could be explained by their acidophilic character. Ogunbanwo et al. [37] reported that the genus Lactobacillus commonly predominates during food fermentation are the most aciduric of all LAB.After 72 hours of fermentation, there was growth reduction from 5.8 to 5.2 Log10CFU/g and 5.5 to 5.2 Log10CFU/g for L. brevis G11 and L. brevis G25 respectively. L. buchneriM11, L. cellobiosusM41, L. fermentum N25 and L. fermentumN33 showed an exponential growth until 120 hours of fermentation. For L. buchneri M11 and L. fermentum N25, growth ranged from 3.3 to 7.4 Log10CFU/g and 3.3 to 6.3 Log10CFU/g after 120 hours of fermentation.These results are in agreement with few reports showing that the population of LAB is increasing with the time of fermentation [24, 38, 26].
4. Conclusions
- LAB used in this work have shown their ability to reduce the pH during fermentation. This certainly helped to significantly reduce fungal growth and AFB1 content in Kutukutu during fermentation. With the increasing interest in food safety throughout the world, this LAB cultures with high antifungal and antimycotoxigenic potential could be of immense value in limiting AFB1 in the food.
ACKNOWLEDGEMENTS
- Authors are grateful to the University of Ngaoundere (Cameroon) for support in the form of infrastructural facilities made available for undertaking the present study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML