-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(2): 71-76
doi:10.5923/j.microbiology.20150502.04
Isolation and Identification of Yeasts from the Different Stages of Hulu-mur Fermentation
Abdel Moneim E. Sulieman1, Esra A. M2, Warda S. Abdelgadir3
1Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia
2Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Sudan
3Food Research Centre, Ministry of Science and Technology, Khartoum, Sudan
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Hulu-mur is the major drink prepared for the special occasion of Siam or Sawm Ramadan. The aim of this study was to isolate and identify yeasts from the different stages of hulu-mur fermentation produced using two sorghum varieties (Feterita and Wad akar). 20 samples were obtained from different stages of hulu-mur production (grain flour, malt, fermented dough at zero time, fermented dough after 12 h fermentation with and without starter, fermented dough after 24 h fermentation with and without starter and the end product). The yeast counts of hulu- mur prepared from Feterita sorghum (FSH) were 3.3× 103, 6.1× 104, 3.6×104 and 8.3×105 C.F.U/ml, while yeast counts of Hulu mur prepared from Wad akar sorghum (WSH) were 3.5× 103, 4.3× 105, 6.3×105 and 1.6×106 C.F.U./ml at time intervals of 3, 9, 12 and 24 hours, respectively. Only two genera of yeast were detected, these were Saccharomyces, with a frequency ranged between 40 and 100% in Feterita sorghum dough (FSD) and 80% in Wad akar sorghum dough (WSD) and Candida, with a frequency of 60%.
Keywords: Hulu-mur, Ramadan, Sorghum, Yeast, Fermented dough
Cite this paper: Abdel Moneim E. Sulieman, Esra A. M, Warda S. Abdelgadir, Isolation and Identification of Yeasts from the Different Stages of Hulu-mur Fermentation, Journal of Microbiology Research, Vol. 5 No. 2, 2015, pp. 71-76. doi: 10.5923/j.microbiology.20150502.04.
Article Outline
1. Introduction
- Sorghum is a staple food grain in many semi-arid and tropic areas of the world, notably in Sub-Saharan. More than 35% of sorghum is grown directly for human consumption. The rest is used primarily for animal feed, alcohol production and industrial products [1]. More than 7000 sorghum varieties have been identified [2]; therefore there is a need of their further characterization to the molecular level with respect to food quality. The acquisition of good quality grain is fundamental to produce acceptable food products from sorghum. Sorghum while playing a crucial role in food security in Africa, it is also source of income of house-hold.In the Sudan, sorghum is the most important food crop. It is the staple food of the vast majority of the population and is produced mainly in the central clay plains of the Sudan under rain, with limited amount being produced in the irrigated schemes of Gezira and Rahad and New Half. The annual production of sorghum in Sudan is about 1.4 million tons with cultivated areas ranging from (2.73-6.43) million hectares [3]. The most popular varieties grown in Sudan are Mayo (Milo in USA), Dabar, Safra, Gasabi and Feterita. These are produced mainly in Eastern Sudan in a rain-fed area, with limited amount being produced in. Western Sudan and in the irrigated area at the Gezira Scheme [4]. Many traditional foods are prepared from sorghum grains in the Sudan, such as hulu- mur, abreh, aceda, kisra, Merissa and nasha. Hulu mur, is a sorghum product strongly linked to the holy month of Ramadan. Hulu-mur, is a sorghum fermented product and is a refreshing drink. Hulu-mur is a result of fermentation carried out mainly by lactic acid [5][6]. The aim of the present study was to isolate, identify and characterize the yeasts contribute in sorghum dough used for hulu mur preparation.
2. Materials and Methods
2.1. Preparation of Hulu-mur
- Hulu-mur dough was prepared at laboratory level using two sorghum varieties (Feterita and Wad akar) by a traditional method as described by Dirar [7], in which, equal amounts of malt and grain were used. The malt and the grain were separately milled into fine flour, the flour from un-germinated grain was then cooked directly into a rather thick porridge. The hot porridge was transferred from the cooking pot to another pot in which fermentation and amylolysis will take place. The malt flour was then added to the porridge while the latter was still hot. The two ingredients were thoroughly mixed with a wooden stirrer. During this process, liquefaction of the porridge occurs very rapidly so that within minutes the mixture is already sweetish and fluid even though water has not been added to it. The liquefied mixture was left to ferment in the sun in a large container, leaving head space to accommodate the expected increase in batter volume ensuing from fermentation gases. During fermentation, an selected spices are ground to powder, in addition to the date slurry were mixed into the fermenting batter.After thorough mixing, the whole mixture was further fermented so that the total fermentation time was 24-36 hours. The batter was then become ready for baking; it was sour and sweetish, and had red-brown colour and strong bouquet of malt and spices.
2.2. Sampling
- Twenty samples were obtained from the different stages of hulu-mur production (grain flour, malt, fermented dough at zero time, fermented dough after 12 h fermentation with adding starter and without starter, fermented dough after 24 h fermentation with adding starter and without starter, starter, spices and the end product). In this study starter culture was used in the fermentation of sorghum dough after zero time and left to ferment, beside the samples fermented without starter.
2.3. Yeast Enumeration
- From suitable dilutions of sample 0.1ml was aseptically transferred onto solidified potato dextrose agar containing 0.1g chloramphenicol per one liter of medium to inhibit bacterial growth. The sample was spread all over the plates using sterile bent glass rod. Plates were then incubated at 28ºC for 48 hours as described by Harrigan [8]. Suspected colonies of yeast were re-streaked on sterile PDA plates and incubated at 28ºC for 48 hours. This procedure was repeated three times to obtain pure colonies of yeast to be ready for identification tests.
2.4. Identification of Yeasts
- Pure isolates of yeasts were identified according to Lodder [9]; Barrnett et al. [10] as follows:The microscopic examination test was accomplished for yeasts from growing cultures which were inoculated into 10ml sterile liquid culture medium composed of 20g glucose, 5g yeast extract, 10g peptone and 1000ml distilled water, and the culture was examined microscopically after incubation at 28ºC for 72 hrs. The shapes of the yeasts cells and the form of budding were observed and registered.For sporulation test, smear was prepared according to Harrigan and McCance [11] and stained by 5% malachite green then investigated under the microscope.The ascospore formation (Sporulation) test was carried out according to Barrnett et al. [12], the following media were used:1- Yeast extract, malt extract and glucose peptone agar.2- Acetate agar which was prepared by dissolving 9.8g potassium acetate, 1.0g glucose, 1.2g sodium chloride, 0.7g magnesium sulphate, and 2.5g yeast extract and 20g agar in a liter of distilled water.3- Potato Dextrose Agar (PDA) (Barrnett et al., 1983) [13].Each of the above media was prepared into bottles as slopes after being autoclaved at 121ºC and 1.06kg\cm² for 15 minutes. Each isolate was then inoculated in each slope and incubated at 28ºC for one to four weeks, and then they were examined microscopically for ascospore formation.For the test for growth at 37ºC and 40ºC, YMA media contained (3g yeast extract, 3g malt extract, 10g glucose, 5g peptone and 20g agar in 1000ml distilled water) (McClay et al., 1959) was inoculated with fresh culture of yeast and incubated aerobically at 37ºC and 40ºC [11].For the test for growth on high D-Glucose concentration, 56 grams of D-glucose were dissolved each in 1% yeast extract solution. Then 3% agar was added and was dissolved by boiling, distributed into test tubes and autoclaved for 15minutes at 121ºC and then sloped and inoculated by streaking and at 25ºC for 4 weeks .The growth in slopes was then examined [10]. The fermentation of glucose was carried out by filling test tubes with 10-15 ml of yeast extract broth containing 2% D-glucose with inverted Durham tubes, then autoclaved at 121ºC for 20 minutes. Each test tube was inoculated with a fresh yeast suspension then incubated at 25ºC for 3 weeks, and examined frequently for bubbles of gas [10].For assimilation of carbohydrates, small test tubes, each containing 10ml sterile medium composed of 0.5% peptone water with 4% test sugar were used. The tubes were inoculated with the selected yeast cultures in duplicate. A Vaseline- paraffin (Vaspar) layer, 2cm deep, was added on the top surface of one tube of the medium. The culture was incubated at 25ºC for four to five days. Fermentation was detected by the lifting of the Vaspar layer [14].Urea broth medium (Difco) was used for urea hydrolysis test, in which, urea broth was dispensed into tubes aseptically, in aliquots of 5 ml then autoclaved at 121ºC for 20 minutes. A loop full of cells from one or two day old culture was suspended in the broth and incubated at 37ºC. The tubes were examined every half hour for a change of color to red which indicates urease activity [10].The Cycloheximide resistance test was similar to the glucose fermentation test with the addition of cycloheximide to the medium (0.1%) and examining for resistance of yeast to cycloheximide [10].
3. Results and Discussion
3.1. Results
- The count of yeasts of the Hulu-mur samples is shown in Table 1. The yeast counts of Hulu mur prepared from Feterita sorghum (FSH) were 3.3× 103, 6.1× 104, 3.6×104 and 8.3×105 C.F.U/ml, while yeast counts of Hulu- mur prepared from Wad akar sorghum (WSH) were 3.5× 103, 4.3× 105, 6.3×105 and 1.6×106 C.F.U./ml at time intervals of 3, 9, 12 and 24 hours, respectively.
|
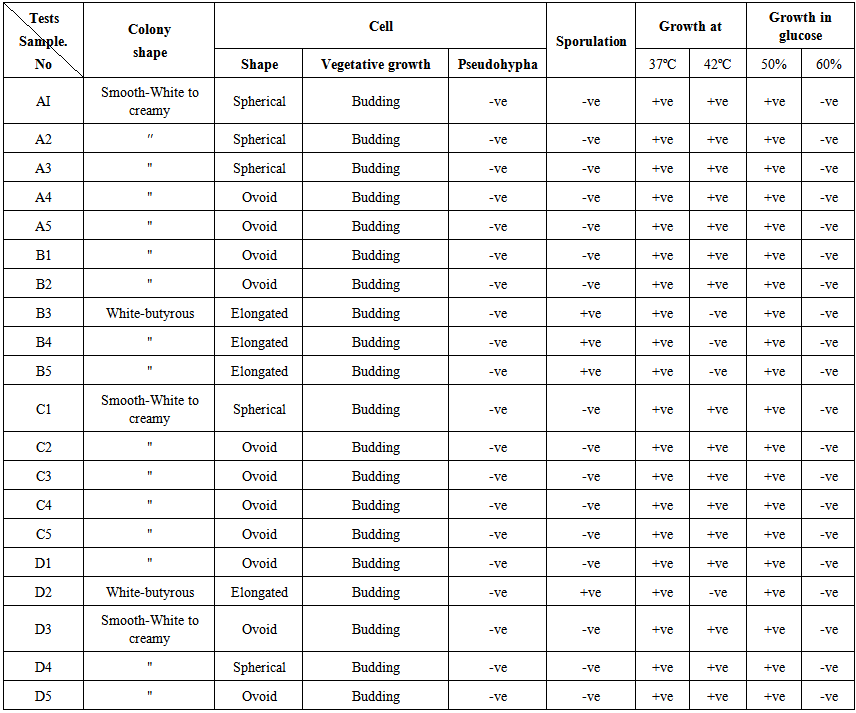 | Table 2. Morphological characteristics and biochemical tests for identification of yeast isolates from different stages of hulu-mur production by using tow sorghum variety (Feterita & Wad Akar) |
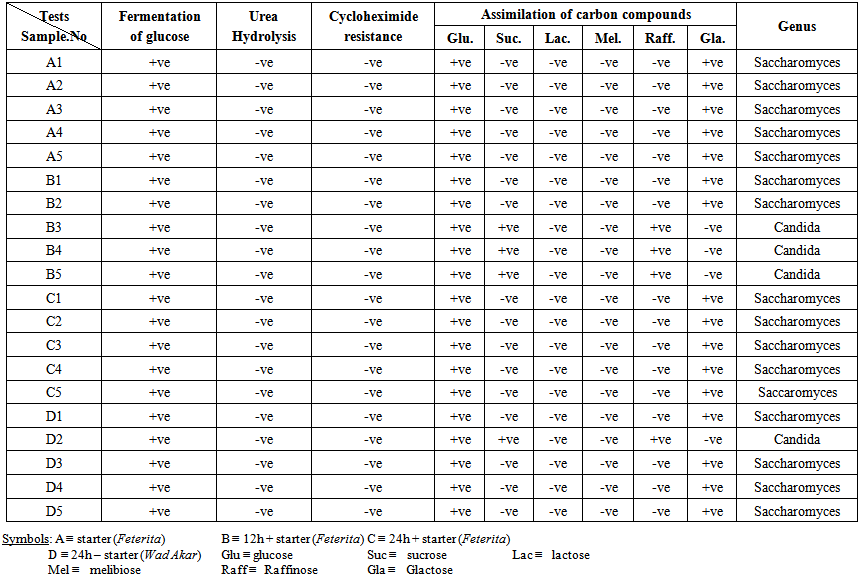 | Table 3. Continuation of identification of yeasts |
3.2. Discussion
- Yeasts are eukaryotic microorganisms classified in the kingdom Fungi, with 1,500 species currently described (estimated to be only 1% of all fungal species). Yeasts are unicellular, although some species with yeast forms may become multicellular through the formation of a string of connected budding cells known as pseudohyphae, or false hyphae, as seen in most molds. Yeast size can vary greatly depending on the species, typically measuring 3–4 µm in diameter, although some yeasts can reach over 40 µm. Most yeasts reproduce asexually by mitosis, and many do so by an asymmetric division process called budding [15].The microbiological analysis revealed high counts of yeasts in the tested Hulu-mur samples which indicated improper hygiene measures during its preparation. These counts were higher than those reported by Sulieman et. al. [16] who found that yeast and mould were devoid in some samples of Hulu-mur while few numbers were detected in other samples (1x102 CFU/ml).Morphological characteristics tests (Table 2) indicate that 70% of the colonies were Smooth-White to creamy and the rest were white-butyrous. 55% of colonies were ovoid while 25% were spherical and 20% were elongated. All isolates reproduced by budding, did not form pseudohyphae and only 25% were spore-former. All yeast isolates were able to grow at 37ºC and 80% were able to grow at 42ºC. All yeast isolates were able to grow at 50% glucose level.The biochemical tests results (Table 3) indicate that all yeast isolates fermented glucose did not hydrolyzed urea and did not resist Cycloheximid. However, all isolates assimilated glucose, but did not assimilated either lactose or melibiose. On the other hand, only 4 isolates (20%) assimilated raffinose and 16 isolated (80%) assimilated glactose.In previous studies [17] isolated five yeasts beside 41 bacterial strains from the various stages of Hulu-mur fermentation. Naturally, the possible role played by the spices in Hulu-mur fermentation initiates the cottage food processors to add these spices after fermentation as they are aware of the inhibitory and stimulatory effects to enhance the growth of certain microorganisms and hinder the growth of others [7]. Agab [17] found that addition of spices to the fermenting batter stimulated the growth of Corynebacterium, Bacillus, Actinobacillus and at a later stage, yeasts. The effect of spices on microorganisms and their activities is well known in other foods of the [18-21].Few studies have been performed on identification and characterization of yeasts isolated from African sorghum beverages. Demuyakor and Ohta [22] reported Saccharomyces cerevisiae as being the predominant species (33%) in Ghanaian pito, but also a high prevalence of Candida spp. (17%) and Kluyveromyces spp. (23%), along with members of six other genera was found. Sanni and Lo¨nner [23] examined Nigerian sorghum beer and found various species, with a dominance of two or more species of either Candida spp., Geotrichum candidum, S. cerevisiae, Kloeckera apiculata or T. delbrueckii, depending on the type of beer.Saccharomyces is a genus in the kingdom of fungi that includes many species of yeast. Saccharomyces is from Greek (sugar) and. Many members of this genus are considered very important in food production. It is known as the brewer's yeast or baker's yeast [24]. They are unicellular and saprophytic fungi. One example is Saccharomyces cerevisiae, which is used in making wine, bread, and beer. Other members of this genus include Saccharomyces bayanus, used in making wine, and Saccharomyces boulardii, used in medicine. Colonies of Saccharomyces grow rapidly and mature in three days. They are flat, smooth, moist, glistening or dull, and cream to tannish cream in color. Generally, they have a diameter of 2-8μ and length of 3-25μ. Blastoconidia (cell buds) are observed. They are unicellular, globose, and ellipsoid to elongate in shape. Multilateral (multipolar) budding is typical. Pseudohyphae, if present, are rudimentary. Hyphae are absent. The fermentation of beer was caused by yeast present in the environment, predominantly the Saccharomyces cerevisiae [25]. Fermentation refers to the procedure of converting the glucose into ethyl alcohol and carbon dioxide gas. In bread production, a different strain of Saccharomyces cerevisiae causes the bread to rise. The bread-making strain is specifically selected, as it produces more carbon dioxide and much less alcohol, which evaporates during baking after all. This genus in this study was more predominant than Candida (figure 15 to 18). It was isolated from four treatments, namely A(100%), B(40%), C(100%) and D(80%). Candida is a genus of yeasts. Many species are harmless commensals or endosymbionts of hosts including humans, but other species, or harmless species in the wrong location, can cause disease. Candida albicans can cause infections (candidiasis or thrush) in humans and other animals, especially in immunocompromised patients [26]. In winemaking, some species of Candida can create potential faults in wines. Many species are found in gut flora, including C. albicans in mammalian hosts, whereas others live as endosymbionts in insect hosts. Antibiotics promote yeast infections, including gastrointestinal Candida overgrowth, and penetration of the GI mucosa. In our study the yeast genus Candida was isolated from only two treatments, B (60%) and D(20%).
4. Conclusions
- Two genera of yeast were detected in the various stages of Hulu-mur fermentation. These were Saccharomyces, its frequency ranged between 40 and 100% in variety Feterita and 80% in Wad akar and Candida, its frequency was 60% in Fetrita and 20% in Wad akar. In conclusion, it is preferably to produce hulu-mur from Wad akar sorghum variety than Feterita variety because all isolated genera was found in wad akar, in addition, it has more frequency of hetrofermentative bacteria that imparts a pleasant flavor and aroma to the fermented dough. It highly recommended to produce hulu-mur by the traditional procedure without adding starter culture, and left the dough to ferment by the natural microflora and the malt enzymes, this is because in order not to lose the natural flavor.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML