-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(2): 57-70
doi:10.5923/j.microbiology.20150502.03
Extended Spectrum Beta Lactamase Producing Strains of Salmonella species - A Systematic Review
Adamu Ishaku Akyala1, 2, Selwa Alsam1
1Department of Biological Science, University of Essex Wivenhoe Park Colchester, UK
2Department of Biological Science, Microbiology Unit, Nasarawa State University, Keffi, Nigeria
Correspondence to: Adamu Ishaku Akyala, Department of Biological Science, University of Essex Wivenhoe Park Colchester, UK.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Salmonella infections in humans can range from a self-limited gastro-enteritis usually associated with non-typhoidal Salmonella(N T S) to typhoidal fever with complications such as a fatal intestinal perforation . In Nigeria, like other developing countries, it is difficult to evaluate the situation of salmonellosis. This is mainly because of the very limited scope of studies, lack of coordinated epidemiological surveillance system and inadequacy of laboratory facilities for culture. In addition, under reporting of cases and the presence of other diseases considered to be of high priority may have overshadowed the problem of salmonellosis in some countries, including Nigeria. Salmonellosis causes significant morbidity and mortality in both humans and animals and has a substantial global socioeconomic impact. Extended-spectrum β-lactamases (ESBLs) are a rapidly evolving group of β-lactamases which share the ability to hydrolyze third-generation cephalosporins and aztreonam yet are inhibited by clavulanic acid. Typically, they derive from genes for TEM-1, TEM-2, or SHV-1 by mutations that alter the amino acid configuration around the active site of these β-lactamases. This extends the spectrum of β-lactam antibiotics susceptible to hydrolysis by these enzymes. An increasing number of ESBLs not of TEM or SHV lineage have recently been described. The presence of ESBLs carries tremendous clinical significance. The ESBLs are frequently plasmid encoded. Plasmids responsible for ESBL production frequently carry genes encoding resistance to other drug classes (for example, aminoglycosides). Therefore, antibiotic options in the treatment of ESBL-producing organisms are extremely limited. In common to all ESBL detection methods is the general principle that the activity of extended-spectrum cephalosporin's against ESBL-producing organisms will be enhanced by the presence of clavulanic acid. ESBLs represent an impressive example of the ability of Gram-negative bacteria to develop new antibiotic resistance mechanisms in the face of the introduction of new antimicrobial agents.
Keywords: Extended, Spectrum, Beta, Lactamase, Salmonella, Strains
Cite this paper: Adamu Ishaku Akyala, Selwa Alsam, Extended Spectrum Beta Lactamase Producing Strains of Salmonella species - A Systematic Review, Journal of Microbiology Research, Vol. 5 No. 2, 2015, pp. 57-70. doi: 10.5923/j.microbiology.20150502.03.
Article Outline
1. Introduction
- Non-typhoidal Salmonella is one of the principal causes of food poisoning worldwide with an estimated annual incidence of 1.3 billion cases and 3 million deaths each year (Tassios et al., 1997). Non-typhoidals fever, which is caused mainly by Salmonella typhimurium and Salmonella enteritidis, continues to be a major problem in developing countries. A recent study estimated that globally there are more than 22 million cases of typhoid fever each year with more than 200,000 deaths, however, the true magnitude is difficult to quantify because the clinical picture is confused with many other febrile illnesses and most typhoid endemic areas lack facilities to confirm the diagnosis (Crump et al., 2004).Antibiotic treatment is not required for Salmonella gastroenteritis but is essential for enteric fever, invasive salmonellosis and in patients at risk of extra-intestinal disease. For many years chloramphenicol, ampicillin, and trimethoprim-sulphamethoxazole (cotrimoxazole) were the drugs of choice. In recent years, increasing resistance of Salmonella species to commonly used antimicrobials has become a matter of concern. Of particular concern are those strains that have acquired multiple drug resistance (MDR) against two or more therapeutic agents. Although fluoroquinolones, such as ciprofloxacin and ofloxacin, and extended spectrum cephalosporins, such as ceftriaxone and cefotaxime, have proved to be effective alternatives, resistance to these agents has emerged (Parry, 2003).To track Salmonella infections and disrupt epidemic spread, many nations have established extensive surveillance systems. Typing to the strain level has been an important tool in surveillance and outbreak investigation of Salmonella infections. Most of these surveillance projects rely on traditional (phenotypic) methods like serotyping, phage and biotyping, which provide a limited means of distinguishing epidemic from endemic or sporadic isolates. Nowadays, phenotypic methods are either replaced or complemented by more sensitive and discriminative molecular techniques. Typing schemes based on variation in particular DNA sequences are digital and the same results could be achieved wherever the test is performed. Sequence based typing schemes can also be considered as genetic classification schemes (Liebana, 2002; Winokur, 2003).
1.1. The Genus Salmonella
1.1.1. General Characteristics
- Members of the genus Salmonella are ubiquitous pathogens found in humans and livestock, wild animals, reptiles, birds, and insects. Salmonellae are Gram-negative, non- spore forming, facultative anaerobic bacilli, 2 to 3 by 0.4 to 0.6 µm in size. Like other members of the family Enterobacteriaceae, they produce acid on glucose fermentation; reduce nitrates to nitrite, and don’t produce cytochrome oxidase (Farmer, 1995). Most organisms except S. gallinarum–pullorum are motile by Peritrichous flagella. The differential metabolism of sugars can be used to distinguish some Salmonella serotypes, e.g., most don’t ferment lactose. S. typhi is the only organism that does not produce gas in sugar fermentation. Salmonella are non- capsulated except S. typhi, S. Paratyphi C and some strain of S. Dublin (WHO, 2003).
1.1.2. Antigenic Structures
- The typical Salmonella are defined mainly by two sets of antigens, somatic (O) and Flagellar (H) that are readily demonstrable by serological reactions in the laboratory. In addition, other bacterial antigens are also available. These include: an exopolysaccharide (Vi, or virulence antigen), the mucus (M), and the fimbrial (F) antigens (Huckstep, 1962; Old, 1996).
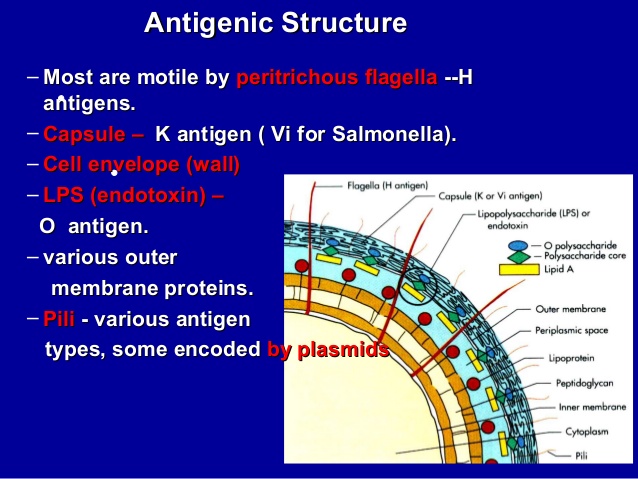 | Figure 1. Antigenic structure of Salmonella species (Adapted from Huckstep, 1962) |
2. Classification of Salmonella
- The classification of Salmonella is complex because the organisms are a continuum rather than a defined species. The current classification of the genus Salmonella is based on DNA-DNA hybridization studies (Crosa et al., 1973). This work suggested that the genus Salmonella is divided into two species known as Salmonella bongori and Salmonella enterica. S. enterica can be further divided into 6 distinct subspecies (enterica, salamae, arizonae, diarizonae, houtenae, and indica), based on different biochemical profiles, in addition to genetic relatedness (Brenner et al., 2000). The majority of the 2500 known serovars that causes disease in warm-blooded animals are found in Salmonella enterica subspecies enterica as shown in Figure 2 (Langridge et al., 2008).
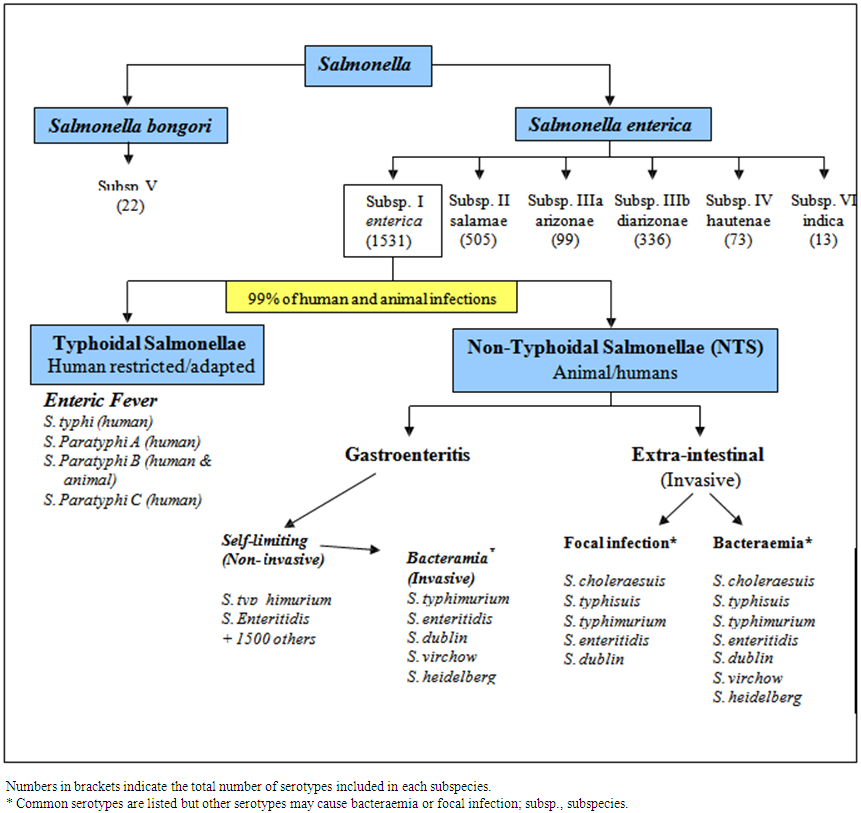 | Figure 2. Classification of the genus Salmonella. (Adapted from Langridge et al. 2008) |
2.1. Salmonella Nomenclature
- Salmonella nomenclature is complex, since different scientists use different systems to refer and communicate about this genus. Some individuals prefer Kauffman’s initial ‘one serovar-one species’ concept, while others favor schemes based on clinical presentation, biochemical characteristics or genetic relatedness (Langridge et al.2008)).According to the Center for Disease and Control (CDC) system, the genus Salmonella contains two species, each of which contains multiple serotypes. CDC uses a name for serovars in subspecies I (for example. serovar Salmonella typhimurium) and uses antigenic formulas for unnamed serotypes (Brenner et al., 2000). At first citation of a serotype the genus name is given followed by the word ‘serovar’ or the abbreviation ‘ser’ and then the serotype. For example, Salmonella serotype or ser Salmonella typhimurium subsequently the name may be written with the genus followed directly by the serotype (for example, Salmonella typhimurium (Popoff et al., 1998).
2.2. Genome of Salmonella
- Because of the presence of large number of serovars in Salmonella, the genome sequence projects concentrated on serovars that are either of importance to human disease or a representative of a particular branch of the Salmonella (Mastroeni, 2006). Genome information can be used to gain insights into the evolution of the Salmonella genus, to identify stable regions conserved between different Salmonella species and serovars, and to identify regions that appear to be specific for individual serovars (Mastroeni, 2006).DNA sequence comparison between the genome of S. typhimurium LT2 and S. typhimurium CT8 show a median homology of 98% (McClelland et al., 2001). Comparison of the genes required for DNA replication, transcription, translation and central metabolism (‘housekeeping’ genes) of the Salmonella serovars indicates that they are extremely similar at the DNA level. Pair wise comparisons between any of the sequenced Salmonella genomes indicate that the similarity between housekeeping genes ranges from 97.6% to 99.5% at the DNA level (Edwards et al., 2002).The complete genome sequence was determined for a multidrug-resistant strain of S. enterica serotype Salmonella typhimurium (CT18). The CT18 genome harbors 4,809,037 base pairs with an estimated 4599 coding sequences. Significant homology has been seen among genomes of S. Typhi-CT18, Salmonella typhimurium LT2 (McClelland et al., 2001) and E. coli K12 (Blattner et al., 1997) genomes, indicating a common evolutionary origin.Salmonella typhimurium is also a member of sub-species I and is the leading cause of gastroenteritis in humans and unlike S. typhi can infect mice and cause a typhoid-like disease. The genome size of Salmonella typhimurium LT2 is 4,857 Kbp (McClelland et al., 2001) and sequence comparison between these two organisms revealed 89% of S. Salmonella typhimurium LT2 CDSs were homologous to S. typhi CT18 at the nucleotide level (McClelland et al., 2001). Although genetically similar to the E. coli K12 genome, both S.typhimurium and Salmonella typhimurium have acquired large regions of extraneous DNA by horizontal transfer known as pathogenicity islands, which offer a selective pathogenic advantage to the organisms (Marcus et al., 2000).Salmonella enterica strains contain plasmids which vary in size from 2 to more than 200 kb. There are different types of plasmids in Salmonella and the best described groups of plasmids are the virulence plasmids (50–100 kb in size) present in serovars enteritidis, Salmonella typhimurium Dublin, Cholerae-suis, Gallinarum, Pullorum and Abortus-ovis. The virulence plasmids have a common 8kb DNA region encoding the spv (Salmonella plasmid virulence) gene involved in intracellular macrophage survival of Salmonella.(Gulig et al., 1993). Depending on serovar these plasmids code for additional virulence- associated genes such as rck (resistance to complement killing), pef (plasmid encoded fimbriae), srgA (SdiA-regulated gene, putative disulphide bond oxidoreductase) or mig-5 (macrophage-inducible) gene coding for putative carbonic anhydrase) (Rychlik et al., 2006).Another group of high molecular weight plasmids are responsible for antibiotic resistance. The antibiotic resistance genes are often located within transposons which can transpose from plasmids to chromosome, and vice versa (Figure 3). Resistance genes can be also found in a form of gene cassettes incorporated into integrons (Hall and Collis, 1995). Small ColE1-type plasmids (pC) of 3-5.6kb have been found in S. enterica serovar Enteritidis and one of these plasmids carries an active restriction modification system, which could explain the high resistance of pC-carrying S. enterica serovar Enteritidis strains to phage infections (Gregorova et al., 2002).
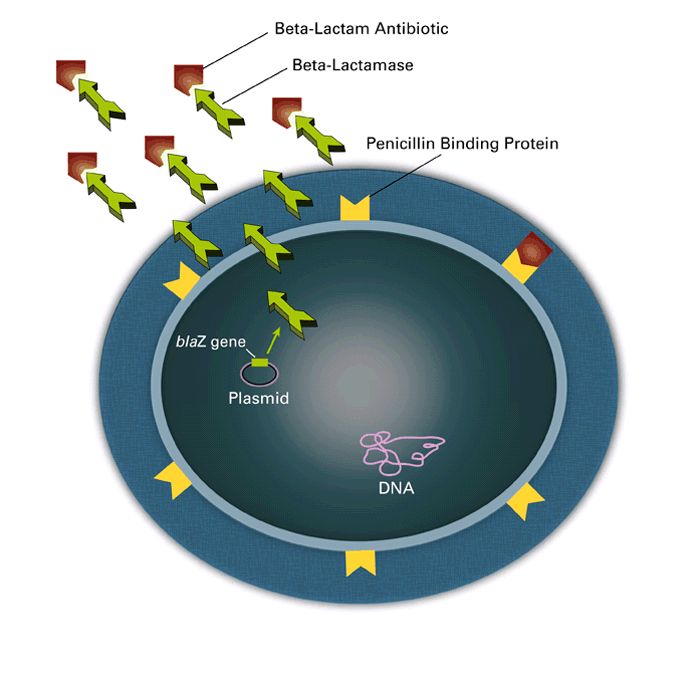 | Figure 3. Schematic representation of plasmid-mediated horizontal transfer of antibiotic resistance |
3. Salmonellosis (The Disease)
3.1. Pathogenesis
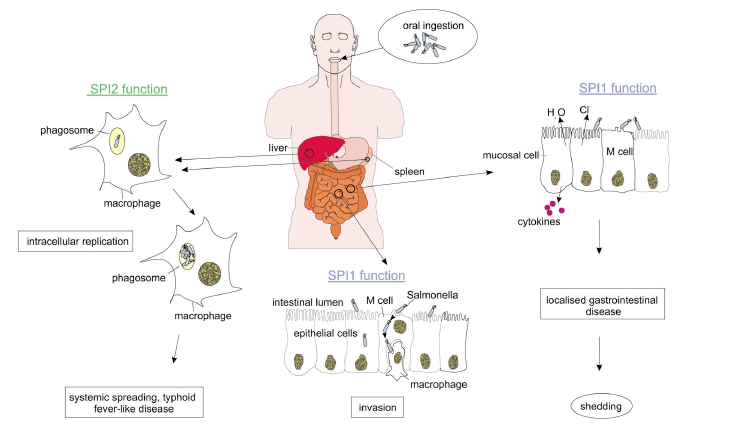
- Salmonellosis in the human host is generally associated with Salmonella enterica subspecies enterica (also termed subspecies I) and acute infections can present in one of four ways: enteric fever, gastro-enteritis, bacteraemia, and extraintestinal (EI) focal infection. As with other infectious diseases the course and outcome of the infection are dependent upon a variety of factors including inoculating dose, immune status of the host and genetic background of both host and infecting organism (Cammie and Miller, 2000).Broadly speaking the Salmonella enterica from human infections can be subdivided in to two groups: the enteric fever (typhoidal) group and non-typhoidal Salmonella (NTS), which typically cause gastro-enteritis but can cause invasive disease under certain conditions. There are five serotypes of Salmonella associated with enteric fever: Salmonella enterica subspecies enterica serovar Salmonella typhimurium, S. Paratyphi A, S. paratyphi B, S. paratyphi C and S. Sendai. S. typhi forms a genetically homogenous group as do and S. paratyphi A and Sendai together, whereas S. paratyphi B and C are heterogeneous (Selander et al., 1990).All Salmonella infections begin with the ingestion of organisms in contaminated food or water. After leaving the stomach, Salmonella must traverse the mucosal layer overlaying the epithelium of the small intestine. After crossing the mucosal layer overlaying the intestinal epithelium, Salmonella interacts with both enterocytes and Microfolds cells (M cells) (Francis et al., 1992). The organisms are rapidly internalized and transported into submucosal lymphoid tissue where they may enter into systemic circulation. Salmonella have also the ability to induce non phagocytic epithelial cells by a process known as bacterial mediated endocytosis. This process involves the formation of large membrane ruffles around the organism and cytoskeleton rearrangement (Francis et al., 1992). Salmonella is then internalized within bound vacuoles through which organisms’ trancytose from the apical to the basolateral surface (Rathman et al., 1997). Once it crosses the intestinal epithelium, Salmonella serotypes that cause systemic infections enter macrophages, and migration of infected macrophages to other organs of reticulo endothelial systems probably facilitates the dissemination of bacteria in the host (Figure 4).
3.2. Virulence Factors
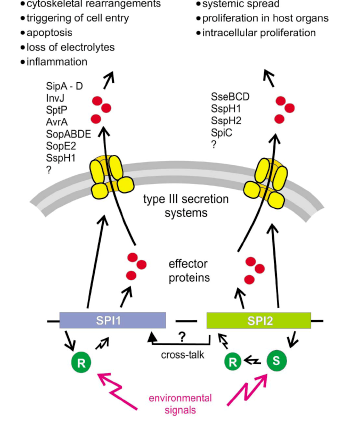
- The outcome of a Salmonella infection is determined by the status of the host and status of the bacterium (Figure 4). The status of the bacterium is determined by the so- called virulence factors (van Asten and van Dijk, 2005).a. Salmonella Pathogenisity Islands (SPIs)The majority of virulence genes of Salmonella are clustered in regions distributed over the chromosome called Salmonella pathogenicity islands (McClelland et al., 2001). The SPIs are of major importance for the virulence of S. enterica. Hallmarks of Salmonella virulence, such as cell invasion, intracellular survival and the production of Vi antigens capsule are encoded by SPIs. Until recently more than 10 SPIs have been identified on the Salmonella chromosome, but SPI-1 and SPI-2 is the central for pathogenesis of Salmonella infections (Hansen-Wester and Hensel, 2001).All types of S. enterica have two large clusters of genes known as Salmonella Pathogenicity Island 1 and 2. Salmonella Pathogenicity Island 1 encodes genes necessary for invasion of intestinal epithelial cells and induction of intestinal secretory and inflammatory response (Galyov et al., 1997). Salmonella lacking a functional SPI-1 Type III secretion system are unable to invade epithelia cells and induce cytokine synthesis (Hobbie et al., 1997). During invasion of the gut, the SPI-1 encoded SipB protein triggers the activation of intracellular Caspase-1 within resident macrophages that induces apoptosis in the infected macrophages resulting in escape of Salmonella from these cells (Hersh et al., 1999). SPI-1 also encodes an effector protein SopB which is an inositol phosphate phosphatase and its enzymatic activity results in activation of chloride channel in the membrane of epithelial target cells leading to the secretion of chloride and loss of fluid into the intestinal lumen (Norris et al., 1998).Salmonella Pathogenicity Island 2 encodes genes essential for intracellular replication and necessary for establishment of systemic infection beyond the intestinal epithelium (Hensel, 2006). The function of the SPI-2 encoded Type III secretion system is required to protect the pathogens within the Salmonella containing vacuole (SCV) against the effectors functions of innate immunity. It has been reported that SPI-2 prevents co- localization of the phagocyte oxidase (Vazquez-Torres et al., 2000) and the inducible nitric oxide synthtases to the SCV (Chakravortty et al., 2002). As a consequence, intracellular Salmonella are protected against damage by reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Boonmar et al., 1998) and against a potent antimicrobial activity of peroxynitrite, which is generated by reaction of reactive nitrogen species and reactive oxygen species (Chakravortty et al., 2002). SPI-2 genes enabled S. enterica lineages to establish a new niche as an intracellular pathogen in the intestinal mucosal and systemic tissue.b. Type III secretion systemsCentral to the pathogenesis of S. enterica is the function of specialized protein secretion systems, known as Type III secretion system (TTSS). TTSS are specialized virulence devices that have evolved indirect translocation of bacterial virulence proteins into the host cell cytoplasm. Type III secretion systems are composed of several proteins that form a remarkable needle-like organelle in the bacterial envelope (Galan, 1998). So far the presence of two SPIs (SPI-1 and SPI-2) each encoding a TTSS, have been described for Salmonella species and may reflect the flexibility of this highly successful pathogen in causing different forms of diseases. SPI-1 is not present in E. coli isolates, this suggest that the acquisition of SPI-1 by Salmonella was a fundamental step in the divergence of these two genera (Hansen-Wester and Hensel, 2001).
3.3. Immunity
- The immune response in Salmonella includes innate and adaptive immunity. The intestinal epithelium, neutrophil, macrophage, dendritic cell, NK cell, and γδT cells take important part in innate immunity and antigen specific T and B cells take part in the adaptive immunity (Mizuno, 2004).The following are some early defense mechanisms in the gut: a) gastric acid may directly kill the bacteria or activate the proteolytic activity of pepsin which is required for the cleavage of Histone 2A, into antibacterial peptide (Mastroeni, 2006); b) phagocyte and innate immunity: usually phagocytic cells control the growth of S. enterica in the first few days of the infection using reactive oxygen species generated via the phagocyte NADPH- oxidase (Mastroeni et al., 2000), while RNS, that are produced following the activation of the inducible nitric oxide synthase, play a role in resistance in the later stage of infection of S. enterica (Mastroeni et al., 2000); c) Cytokines are key regulators of the host responses in intracellular pathogenesis and various bacterial products from Salmonella are potent inducers of cytokine expression by immune cells (Lalmanach and Lantier, 1999); d) antibody response to S. enterica infections induce early IgM antibody responses followed by IgG and IgA production (Mastroeni, 2006).A large number of antigens including LPS determinants (O-polysaccharide and core regions), Vi antigen, porins, outer membrane proteins, lipoproteins, heat shock proteins, flagella and fimbriae are recognized by Salmonella-specific antibodies (Mastroeni, 2002); e), T cell response: S. enterica infections induce proliferation of CD4+ T cells which plays a pivotal role in activation of macrophage (Mastroeni, 2002). CD8+ T cells is also involved in producing IFN-γ and lysing target cells infected with S. enterica (Salerno-Goncalves et al., 2003). Suppression of the growth of S. enterica is followed by the elimination of the bacteria from the tissue. If the bacteria are not cleared, a late resurgence of bacterial growth can occur (relapse), or a chronic carrier state can develop, which can be a serious problem since it constitutes a reservoir of infection (Mastroeni, 2006).
4. Mechanism of Drug Resistant
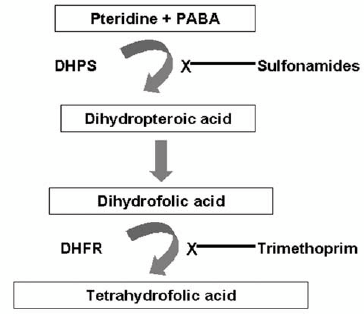
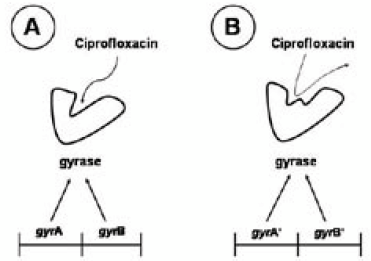
- Efforts aimed at identifying new antibiotics were once a top research and development priority among pharmaceutical companies. The potent broad spectrum drugs that emerged from these endeavors provided extraordinary clinical efficacy. Success, however, has been compromised. We are now faced with a long list of microbes that have found ways to circumvent different structural classes of drugs and are no longer susceptible to most, if not all, therapeutic regimens (Alekshun and Levy, 2007).Resistance to various classes of antimicrobial agents has been encountered in many bacteria of medical and veterinary relevance. Over the years, various studies have reported the presence of genes and mutations conferring resistance to antimicrobial agents in zoonotic bacteria such as Salmonella (Michael et al., 2006).There are three major mechanisms by which bacteria have become resistant to antimicrobial agents: enzymatic inactivation; reduced intracellular accumulation of the antimicrobials; protection, alteration or replacement of the cellular target sites (Schwarz and Chaslus- Dancla, 2001).a. ChloramphenicolChloramphenicol (CAF) is a broad-spectrum bacteriostatic agent and the antibacterial action of chloramphenicol is mediated by inhibiting protein synthesis after binding to the 50S subunit of the bacterial ribosome and thus preventing the transfer of the new amino acid from its tRNA to the growing peptide chain. This binding is achieved by molecular mimicry of the peptidyl adenyl terminus of the tRNA molecule (Schwarz et al., 2004). The first and still most frequently encountered mechanism of bacterial resistance to CAF is enzymatic inactivation by acetylation of the drug via different types of chloramphenicol acetyltransferases (CATs) (Murray and Shaw, 1997). However, there are also reports on other mechanisms of CAF resistance, such as efflux systems, inactivation by phosphotransferases, mutations of the target site and permeability barriers (Murray and Shaw, 1997).There are two defined types of CATs which distinctly differ in their structure: CatA and CatB enzymes. In Salmonella, enzymatic inactivation by type A or type B chloramphenicol acetyltransferases (Cat) as well as the export of chloramphenicol or chloramphenicol/florfenicol by specific efflux proteins is the dominant resistance mechanisms (Michael et al., 2006). Two different types of CatA proteins, encoded by the genes catA1 and catA2, have so far been detected in Salmonella isolates. While the Tn9- borne resistance gene catA1 has been found in several serovars, including Salmonella typhimurium (Chen et al., 2004), the gene catA2 was detected on a multiresistance plasmid from Salmonella typhimurium, and Salmonella typhimurium (Randall et al., 2004)).Three different types of catB genes, catB2, catB3 and catB8, are known to occur in Salmonella. All these catB genes are located on gene cassettes and have mainly been identified in class 1 multi-resistance integrons in Salmonella typhimurium (Nogrady et al., 2005). The chloramphenicol exporter gene cmlA is also a cassette borne gene which has been found in plasmid-located class 1 integrons in Salmonella typhimurium (Nogrady et al., 2005).b. Co-trimoxazoleA combination of trimethoprim and sulphamethoxazole, known as co-trimoxazole has been successfully used to treat Salmonella infections. Since these two antimicrobials inhibit sequential stages in tetrahydrofolic acid (THFA) synthesis it was believed that administration of a combination therapy would have a selective advantage over a single administration (Nogrady et al., 2005).Sulfonamides and trimethoprim block different enzymatic steps in tetrahydrofolate biosynthesis (Figure 9). Sulfonamides are structural analogs of p-aminobenzoic acid and competitively inhibit the enzyme dihydropteroic acid synthase (DHPS) while trimethoprim competitively inhibits the enzyme dihydrofolate reductase (DHFR) (Sköld, 2001).Chromosomal, plasmid and transposon mediated resistance have all been reported for this antimicrobial. Chromosomal mutations involve the over production of the dihydrofolate reductase (DHFR), which leads to trimethoprim resistance involving the need for a higher inhibitor concentration of drug inside the cell to decrease the residual enzyme activity. The commonest mechanism of trimethoprim resistance is associated with the production of an additional non-susceptible form of DHFR encoded by genes located on self-transmissible or mobile plasmids and transposons (Huang et al., 2004).
5. Conclusions
- Antibiotic resistance is an important issue affecting public health, and rapid detection in clinical laboratories is essential for the prompt recognition of antimicrobial-resistant organisms. Infection-control practitioners and clinicians need the clinical laboratory to rapidly identify and characterize different types of resistant bacteria efficiently to minimize the spread of these bacteria and help to select more appropriate antibiotics. This is particularly true for ESBL-producing bacteria. The epidemiology of ESBL-producing bacteria is becoming more complex with increasingly blurred boundaries between hospitals and the community. E coli that produce CTX-M β lactamases seem to be true community ESBL producers with different behaviors from Salmonella spp, which produce TEM-derived and SHV-derived ESBLs. These bacteria have become widely prevalent in the community setting in certain areas of the world and they are most likely being imported into the hospital setting. A recent trend is the emergence of community-onset bloodstream infections caused by ESBL-producing bacteria, especially CTX-M-producing Salmonella spp. These infections are currently rare, but it is possible that, in the near future, clinicians will be regularly confronted with hospital types of bacteria causing infections in patients from the community, a scenario very similar to that of community-acquired Salmonella spp, The carbapenems are widely regarded as the drugs of choice for the treatment of severe infections caused by ESBL-producing Enterobacteriaceae. The spread of E coli that produce CTX-M β lactamases will have important future implications for the empirical treatment of community-associated bloodstream infections, particularly in patients with associated urinary-tract infections, and therefore merits close monitoring with enhanced surveillance studies. Molecular methods for the detection of CTX-M β lactamases show potential to screen large numbers of these bacteria in a rapid fashion. We recommend that internationally funded efforts should be undertaken to track and monitor the worldwide spread of Salmonella spp, that produce CTX-M β lactamases within the hospital and community settings. If this emerging public-health threat is ignored, the medical community may be forced to use the carbapenems as the first choice for the empirical treatment of serious infections associated with urinary-tract infections that originate in the community. Research is warranted to determine whether significant clinical differences exist among the carbapenems, and to define the best therapy of less severe infections caused by ESBL-producing Enterobacteriaceae. We also recommend that future investigations be undertaken to study the microbiological and ecological factors that make CTX-M-producing Salmonella spp, such successful pathogens. This will help to prevent future infections caused by these medically important pathogens.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML