-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(2): 46-56
doi:10.5923/j.microbiology.20150502.02
Effect of Sheep Diets Containing Microbiological Treated Rice Straw on Blood Parameters and Nitrogen Balance
Mustafa M. ELmoghazy1, Husain M. El-Fadaly2, Tag EL-Din H. Tag EL-Din3, Hamada A. Areda1
1Agric. Animal Production Dept., Fac. of Agric., Damietta University, Damietta, Egypt
2Agric. Microbiology Dept., Fac. of Agric., Damietta University, Damietta, Egypt
3Agric. Poultry Production, Dept., Fac. of Agric., Damietta University, Damietta, Egypt
Correspondence to: Hamada A. Areda, Agric. Animal Production Dept., Fac. of Agric., Damietta University, Damietta, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The objective of this investigation was to verity the potential benefits of microbiological treatments of rice straw as an agriculture by-product produced in huge amount in Egypt to be used in preparation of small ruminants diets. Five microorganisms were used in 17 treatments beside control and fortified control. These microorganisms were selected upon their cellulolytic activities being two bacteria, Bacillus licheniformis (B1), Ruminococcusalbus (B2), fungal strains being Aspergillus oryzae (F1), Rhizopus nigricans (F2) and one yeast strain called Saccharomyces cerevisiae (Y). These microorganisms were used either individually or in a mixture form. The effect of these treatments was examined from chemical point of view of the diets composition and blood parameters as well. The faeces components and nitrogen balance were also considered. Results of the chemical analyses showed that rice straw contained insufficient crude protein being 5.61% that equal to 5.98% of dry matter (93.7%) that not proper for rumen microorganisms and fermentation. Obtained results exhibited that the microbiological treatments significantly (P≤ 0.001) decreased the dry matter from 93.70 to 47.61. The treatment in which five microorganisms were used showed to be the best treatment by 49.18%. Results proved that crude fiber was also significantly (P≤ 0.001) decreased from 33.15 to 27.14 by 18.12% while crude protein was significantly (P≤ 0.001) increased from 5.61 to 11.40 by 103.2%. In case of faeces components, results showed no significant differences for crude protein, ether extract and nitrogen free extract. On the other hand, high significance (P≤ 0.001) with ash values was found while significant (P≤ 0.05) differences were found for dry matter among the three treatments, Control, fortified control and microbiological treatment, T17. Both organic matter and crude fiber were significantly (P≤ 0.01) changed. In addition, faecal nitrogen and nitrogen balance exhibited no significance while high significance (P≤ 0.001) was found in case of nitrogen intake, urinary nitrogen and digested nitrogen as well. Regarding blood parameters, serum albumin (gdl-1) was not affected by microbiological treatments while both plasma total protein (P≤ 0.001) and plasma globulin (P≤ 0.01) significantly changed at the 6th h post feeding among the three treatments, control, fortified control and microbiological treatment (T17). Results also showed that plasma total lipids, plasma total glucose or plasma urea-nitrogen were not affected. Furthermore, both Got (Ast) and Gpt (Alt) were not affected proved the microbiological treatment had no effect on the liver functions.
Keywords: Microbiological treatments, Rice straw, Blood parameters, Ruminococcusalbus,Aspergillusoryza
Cite this paper: Mustafa M. ELmoghazy, Husain M. El-Fadaly, Tag EL-Din H. Tag EL-Din, Hamada A. Areda, Effect of Sheep Diets Containing Microbiological Treated Rice Straw on Blood Parameters and Nitrogen Balance, Journal of Microbiology Research, Vol. 5 No. 2, 2015, pp. 46-56. doi: 10.5923/j.microbiology.20150502.02.
Article Outline
1. Introduction
- Ruminants in many tropical countries subsist mainly on crop residue based diets. The increasing expansion of agro-industrial activity over the last few years has led to the accumulation of a large quantity of lingo-cellulosic residues all over the world. The major agricultural by-products in terms of volumes generated in million metric tons, (MMT) were found to be rice straw (112), rice husk (22.4), wheat straw (109.9), sugarcane tops (97.8) and bagasse (101.3) as repointed by [1]. Although a vast energy potential is locked in these lingo-cellulosic crop residues, these are not utilized to their fullest potential for ruminant feeding due to poor digestibility, low nitrogen and mineral contents which rendered them to be classified under non-main-tenancy type of feeds. Also, the covalent encrustation of plant cell wall with lignin prevents their biodegradation in the rumen. Therefore various physical and chemical treatments have been tried, which are known to improve feed quality either by increasing digestibility or by enhancing palatability. Rumen contains a large population of microbes. The rumen microbial ecosystem comprises at least 30 predominant bacterial species at a total concentration of 1010 to 1011/mL of rumen fluid [2]. Most of these microorganisms degrade cellulolytic materials. It is well known that cellulose is one of the most abundant biopolymers on earth and is an important structural component of the plant cell wall. Bacterial species of the rumen are considered important in determining the extent and rate of feed degradation and utilization for microbial protein production [3]. Since the establishment of effective methods to isolate and cultivate ruminal microbiota, a number of cellulolytic rumen bacteria, such as the genera Fibrobacter, Ruminococcus, Clostridium, Lactobacillus and Prevotella, have been isolated [4]. In addition to F. succinogenes and R. flavefaciens, R. albus is one of the main cellulolytic bacteria in the rumen [2]. In-vitro studies have shown that R. albus became predominant over the other two fibrolytic species in co-cultures containing cellulose as well as in the rumen of cows [5]. Therefore, the objective of this research was to examine the effect of sheep diets containing microbiological treated rice straw on blood parameters. The chemical analyses after microbial treatment with N-balance and faeces fractions were also considered.
2. Materials and Methods
2.1. Experimental Animals
- Nine of rahmany rams were purchased from a local animal market of Kafr Sad, Damietta governorate, Egypt.
2.2. Rice Straw
- Sakha 101 rice straw was obtained from Kafr El Batikh cultivation soil, Damietta governorate, Egypt.
2.3. Molasses
- Molasses was kindly taken from Blkas sugar beet factory, Dakahlia governorate, Egypt.
2.4. Microorganism Used
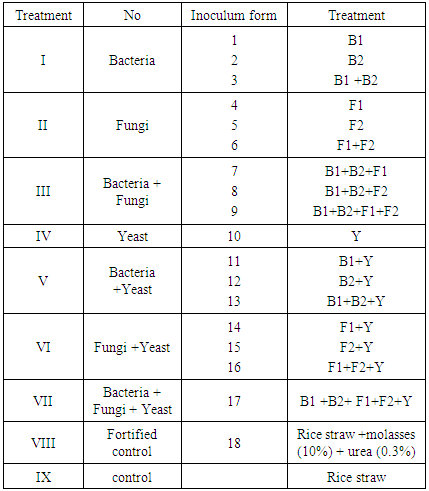
- Five microorganisms were used for treatment of rice straw containing diets. Tow bacterial strains namely Bacillus licheniformis (B1) [6], which isolated and identified from goats. Ruminococcus albus (B2) [7], [10], which isolated and identified from sheep’s, Tow fungal strains namely Aspergillus oryzea (F1) [8] and Rhizopus nigricans (F2) [9] and one yeast strain called Saccharomyces cerevisiae (Y) [10] were used individually and or in a mixture forms in the same ratio. These strain were kindly taken from Microbiology Dept., Faculty of Agriculture, Damietta University, Damietta, Egypt.
2.5. Cultivation Media
- Four liquid cultivation media were used such as milk containing medium, starch containing medium, cellulose containing medium, oil containing medium. These media were used for examining the fermentation potentialities of the rumen bacteria by measuring both length and volume of obtained gas. Both Saccharification, proteolytic, cellulolytic and lipolytic activities were taken in to consideration. Two solid cultivation media were also used for maintained and enumeration of used microorganisms such as nutrient (NA) agar and potato dextrose (PDA) agar media. The composition of those cultivation media were as mentioned in [11].
2.6. Microbial Antagonism
- The plate diffusion methods was used .A standard loop (0.01ml) of each examined bacteria (B1×B2) was put in one side of a petri dish containing NA medium. A standard disk (0.6 cm) of each fungal growth (F1×F2) was put in one side of a petri dish containing PDA medium. The same process was repeated to examine the antagonisms between bacteria – yeast (B1×Y and B2×Y), bacteria- fungi (B1×F1, B1×F2), (B2×F1, B2×F2) and fungi-yeast (F1×Y and F2×Y). All petri dishes were incubated at 37°C for appropriate period of time. At the end of incubation period, the presence of inhibition area was observed [12].
2.7. Preparation of Fortification Solution and Microbial Inocula
- The mixture of used microorganisms was prepared using two different solutions A and B that called microbial fortification solution. For carrying of microorganism the first solution (A) was 10% beet molasses in tap water which autoclaved at 121°C for 30 min. The second one (B) was 0.3% urea in tap water which sterilized by using Millipore filter (Flowpore D 0.2 µ, Made in Germany). So, the mixture ratio was 1:1:1 (v/v/v) for solution A, B and microbial solution fortification prepared in the same ratio.The tow solutions were mixed well and inoculated with an 24 h old bacterial strain and yeast. In case of using fungi, 3 days old cultures were used for inoculation in the same ratio. This inoculum was prepared in 250 ml and used in 1:1 ratio for inoculating 250 gm autoclaved rice straw containing nylon bag (42×25 cm). Before being opened, the incubated materials were physically tested for the structure, smelling, temperature, color and rotting as well before being used in the formulation of the experimental rations as mentioned in Tab.1. [10].
|
2.8. Large Scale Ensiling
- The experiments were prepared by mixing solution (A) and solution(B) as shown before, this volume was completed to 100L then mixed well with 100kg of rice straw. The treated materials were pressed well and covered with plastic sheet holding 100 kg, for each and kept for 4 weeks.
2.9. Chemical Analyses
- Samples of the untreated and microbiologically treated roughages were ground and subjected in triplicates to chemical analyses. Samples of daily output of feces were taken after drying at 80°C to constant weight and then they were also ground. All the ground samples were stored in stoppered brown bottles. Dry matter (DM), crude protein (CP), and crude fiber (CF) of the feedstuffs and faeces samples were determined according to [13].
2.10. In Vivo Experiment
- The experimental design was planned to three groups. The first group was fed on control, the second one was fed on fortified control (control +10% molasses + 0.3% urea) while the third treatment was the microbiological treatment (T17) that including the five microbes used (Tab.1) assigned by B1+B2+F1+F2 and Y in equal ratio. Three Rahmany rams with average live body weight of 45 kg were used in each group. Each rams was housed in an individual metabolic crate. Feed was introduced tow time daily at 7:00 am and 7.00pm. Fresh water was available at all times for all animals. The experimental ration used in the in-vivo study was 40 and 60 for dietary roughage level (DRL) and concentrate feed mixture (CFM) in case of three treatments used; control, fortified control and microbiological treatments (T17). At the end of each experimental period, blood samples were collected in dried clean heparinized tubes by jugular vein puncture from all rams in the morning just before feeding and drinking and immediately centrifuged at 4000 rpm for 15 minutes using PLC-012E Universal Centrifuge. The plasma was carefully taken and stored at -20°C until analysis.
2.11. Blood Parameters
- Blood samples were collected at the end of the collection period from each animal at three times in the morning just before feeding 0.0h then at 3th h and 6th h post feeding. Samples were obtained from the jugular vein through a clean dry needle into 10 ml heparinized test tubes. To assess total protein, albumin, globulin, alanine aminotransferase (ALT, GPT), aspartate aminotransferase (AST, GOT), and urea were measured. All the biochemical constituents of blood serum were calorimetrically measured using a specific kit by the Chemistry Auto-analyzer (Jenway 6315). The activities of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvate transaminase (GPT) were measured as described by [14]. Total protein, was determined according to [15]; Albumin, was determined according to [16], while globulin was calculated by subtracting the albumin value from the corresponding total protein value. Albumin/ globulin ratio was calculated by dividing the albumin value over the corresponding globulin value. Plasma urea was determined using the colorimetric method as described by [17] using commercial kits. Glucose was determined by the modified method of [18].
2.12. Statistical Analyses
- Data were analyzed using the general linear models procedure GLM adopted by [19]. Differences between means were tested for significance using multiple range tests according to [20]. Analysis of variance of repeated measurement, least square means (LSM) and standard errors (SE) were applied using the following statistical model:Yij = µ + Ti + Rj + eijYij is the individual observation of the parameter measured.μ = is the overall mean.Ti = the effect of treatment in each time.Rj = the effect of time in each treatment.Eij = the random error term.
3. Results
3.1. Microbial Antagonism
- Obtained results concerning the antagonism among examined five microorganisms indicated negative result. This means that no interaction or any competition was happened between those five microorganisms when they are co-cultured according to the scheme mentioned in Material and Methods.
3.2. Values of Chemical Components
3.2.1. Dry Matter (DM)
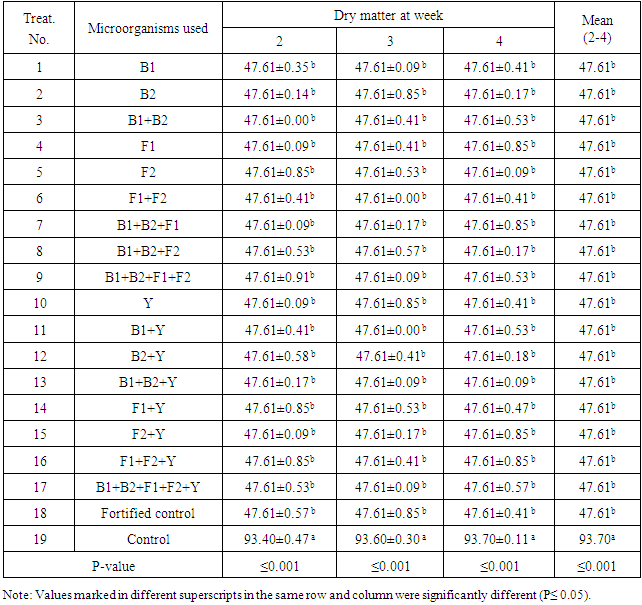
- In order to evaluate the microbiological treatments of the rice straw for small ruminants, the chemical analyses were carried out and obtained results are recorded. Results of dry matter (DM) are listed in Tab.2 showing that all microbiological treatments gave, more or less, the same results to be 47.61 with no significant differences between each other. Typical value was obtained with the fortified control. On the other hand, high significant (P≤ 0.001) difference were found between control and each microbiological and fortified control treatment. Results showed that all values of the microbiological treatments (T17) or the fortified treatment were high significantly (P≤ 0.001) decreased compared with control. The microbiological treatments were conducted on rice straw in 17 treatments giving high significant differences (P≤0.001) on the level of treatments periods being 2,3and 4 weeks as shown in Tab.2.Result showed that the microbiological treatments (1-17) lead to high significant decrease (p≤0.001) to be 47.61% with the treatment of fortified control compared to control. Results also proved that the microbiological treatment (T17) showed to be the same in dry matter (DM) content being 47.61% either after 2, 3 or 4 weeks treatment. This result proved that using the five microbial strains showed to be the same in their fermentation activities either individually or in a mixture form as shown in Table 2.
|
3.2.2. Crude Protein (CP)
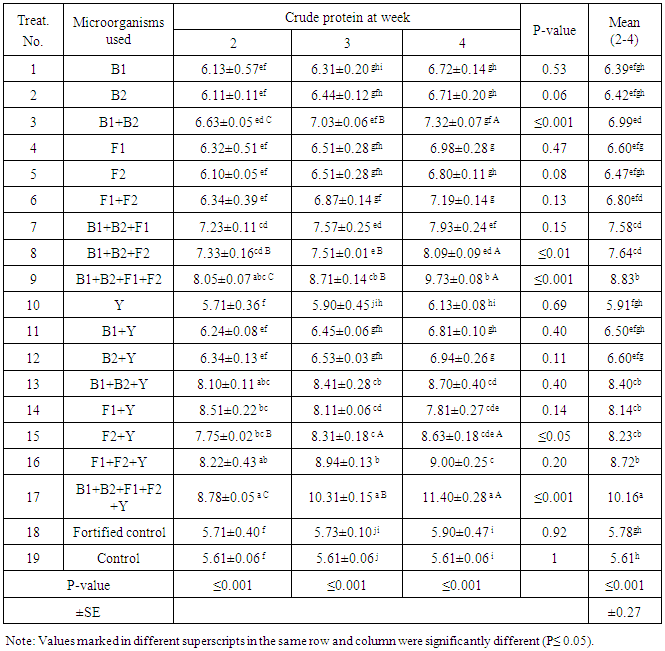
- For crude protein values, result in Tab.3 show high significant differences (P≤0.001) on the level of different treatments, 2, 3 and 4 week. On the other hand, treatment No. 3, 9 and 17 gave high significance (p≤0.001) on the level of treatment period. Additionally, treatments No.8 (P≤0.01) and No.15 (P≤0.05) gave significant different taking the treatment period in to account. Furthermore, the overall mean values showed also high significance (P≤0.001) while T17 showed to be the highest values in crude protein to be 10.16 proved the superiority of this treatment. This treatment is also giving high values of crude protein at all-time tested being 8.78, 10.31 and 11.4% for 2nd, 3cd and 4th week, respectively. No significance was found in other treatments. As mentioned before, rice straw has in sufficient crude protein (CP) of 5.61% that equal to 5.98% of dry matter (DM) which 93.7% that not proper for rumen microorganisms and fermentation. The increase in crude protein can be illustrated by the microbial activities that produce extracellular hydrolytic enzymes which convert complicated molecules in rice straw to fermentable sugars that used as carbon sources for microorganisms to be able to produce biomass which enriching the small ruminants rice straw containing diets.The highest protein values were found after the 4th week with T17 to be 11.4±0.28. Treatment No.17 showed to be the best after the 4th week being 11.4%±0.28 recorded 2.04 and 1.93 fold increase compared to control, and fortified control, respectively. Even so, the obtained values of crude protein (CP) gave apposite trend of dry matter (DM). in addition, the mean value obtained with treatment No.17 (T17) vas 10.16 that increased by 1.8 fold over the control to be 5.61% as can be seen in Table 3.
|
3.2.3. Crude Fiber (CF)
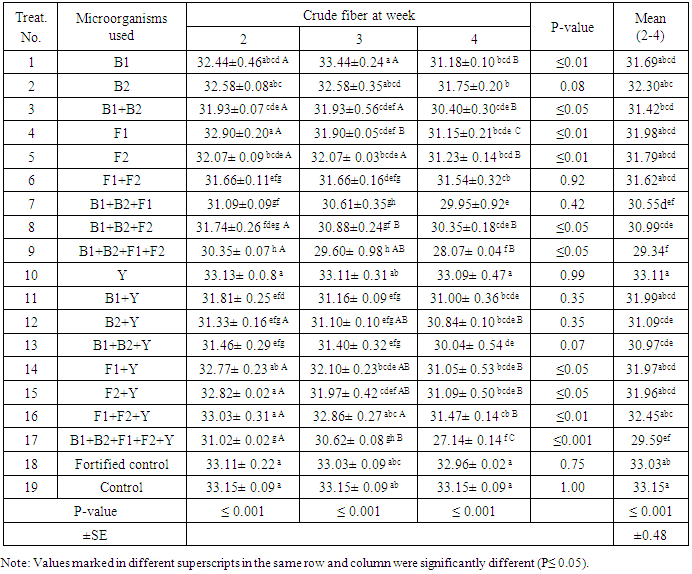
- In case of crude fiber, results in Tab.4 show high significant difference (P≤0.001) amongst three treatments of 2nd, 3rd and 4th week with overall mean value of 29.59. The microbiological treatments leads to decrease the crude fiber values compared to control. The treatment in which five microbes were used gave the highest decrease giving significant differences between control and fortified control as well as between control and treatment of using five microbes (B1+B2+F1+F2+Y).Tabulated results showed that treatments No. 1, 4, 5 and 16 gave significance (P≤0.01) in crude fiber % on level of treatment period while significant (P≤0.05) different were found in case of treatments No. 3,8,14 and 15 as can be seen in Tab.4. The lowest value of crude fiber was found after the 4th week with T17 being 27.14±0.14 with overall mean value of 29.59. This result exhibited the superiority of T17 over other treatments in accordance to crude fiber value.
|
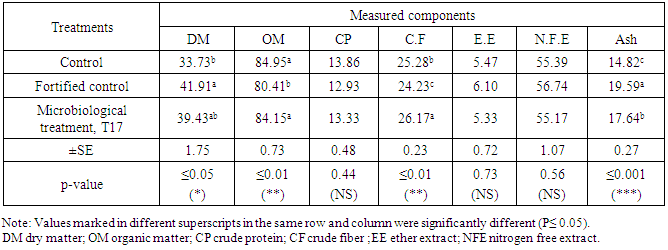
3.3. Faeces Components and Nitrogen Balance
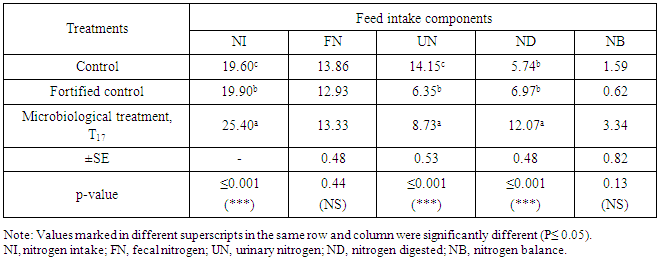
- The chemical analyses of feces were adopted to compare the microbiological treatment (T17) and control as well as the fortified control. Obtained results are listed in Table.5 for DM, OM, CP, CF, EE, NFE and ash. Results showed that DM (P≤0.05), OM (P≤0.01) and CF (P≤0.01) were significant among the three treatments. No significant differences were found in case of CP, EE and NFE while ash gave high significant (P≤0.001) differences among the three treatments, control, fortified control and microbiological treatment T17 which was selected amongst 19 treatments.For N- balance, results in Tab.6 showed high significant (P≤ 0.001) differences in case of NI, UN and ND while no significant differences were found with FN and NB. The microbiological treatment showed to be superior in NI, ND and NB to be 25.4, 2.07± 0.48 and 3.34 ± 0.82, respectively. N-balance proved the importance of microbiological treatment for N-feeding of the tested ruminants that maximized diet usefulness.
|
|
3.4. Blood Parameters
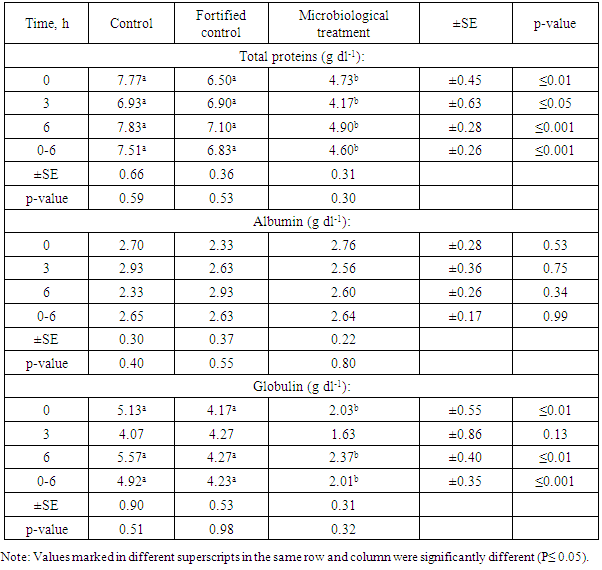
3.4.1. Plasma Total Proteins (PTP)
- Table 7 shows values of total proteins (g dl-1), albumin (g dl-1) and globulin (g dl-1). In case of protein on the level of sampling time, the mean values were 7.51±0.66; 6.83±0.36 and 4.6±0.31 for control, fortified control and microbiological treatment, respectively. Regarding albumin, the mean values were 2.65±0.30, 2.63±0.37 and 2.64±0.22 in the same order, respectively. The mean values of globulin were 4.92±0.9, 4.23±0.53 and 2.01±0.31 in the same order, respectively.
|
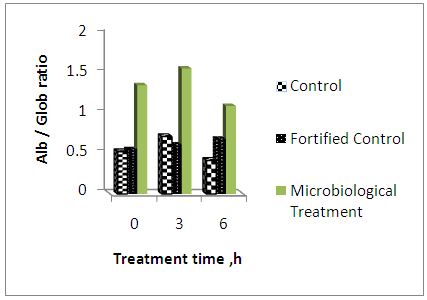 | Figure 1. Values of albumin / globulin ratio against treatment time for the three treatments |
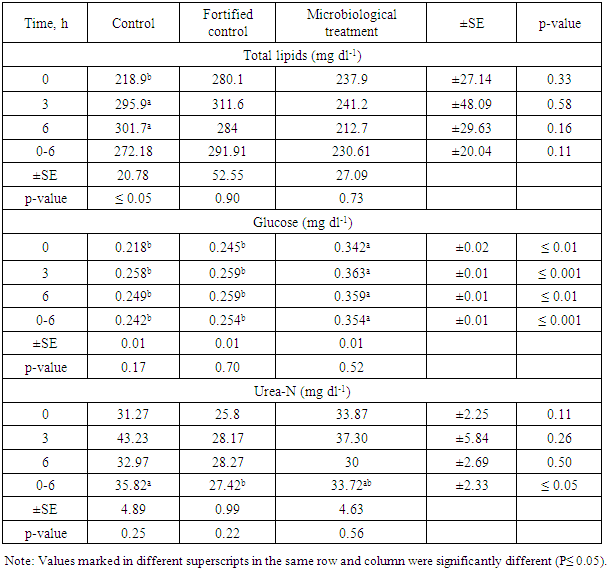
3.4.2. Plasma Total lipids (PTL)
- Table 8. shows values of the plasma total lipids (mg dl-1), glucose (mg dl-1) and urea-N (mg dl-1). For the plasma total lipid, the mean values were 272.18±20.78, 291.91±52.55 and 230.61±27.09 for control, fortified control and microbiological treatment, respectively. Results showed no significant differences among the three treatments. Values of plasma lipids in case of control showed significant differences (P≤0.05), while other tow treatment showed to be insignificant.
3.4.3. Plasma Total Glucose (PTG)
- Regarding plasma total glucose values, no significant differences were found on the level of tested time for each treatment. On the other hand, significant difference was found with 0.0h sample while high significant differences (P ≤ 0.001) were found for 3h sample as well as the mean value. The mean values for glucose were 0.242±0.01, 0.254±0.01 and 0.354±0.01 in the same order, respectively. These values showed significant (P ≤ 0.01) differences as shown in Tab.8.
|
3.4.4. Plasma Urea Concentration (PUC)
- For urea-N, the mean values were 35.82±4.89, 27.42±0.99 and 33.72±4.63 in the above order, respectively. No significance was found among the three tested treatments except the mean value of the three times of samples since it gave significance (P≤0.05) among the three times.
3.4.5. Liver Functions (LF)
- Regarding the enzymatic activities of liver functions, results of GOT (Ast) (mg dl-1) and GPT (Alt) (mg dl-1( are listed in Table 9. The mean values of GOT were 27.44 ± 1.41, 27.66 ± 0.75 and 21.11 ± 3.06 for control, fortified control and microbiological treatments, respectively. Sample of 6h exhibited significant (P ≤ 0.05) difference on the level of treatments while high significance (P ≤ 0.001) was found on the level of overall mean as shown in Tab.9. On the other hand, no significance were found on the level of sampling time for the three treatments. In case of GPT (Alt), sample of 3h gave significant (P ≤ 0.05) difference on the level of treatments. The overall mean value gave high significance (P ≤ 0.001) on the level of treatments while no significantly was found on the level of sampling time.
|
4. Discussion
- Rice straw is fibrous and amatively high lignocellulose [21]. The enhanced DM digestibility driven by the improved fermentation activities of the rumen bacteria, especially cellulolytic strains appeared to increase by supplementing SC, which is capable to scavenge excess oxygen to create optimal environment for rumen anaerobic bacterial activities. Nevertheless, some of S. cerevisiae strains lack the ability to have stimulatory effects on rumen fermentation [8].Autolysis occurs after growth ceases and the degradation of bacterial protein is accompanied by the production of branched-chain amino acids. The organism is strongly proteolytic and protease is produced in the exponential growth phase [22].The high fiber contents are believed to be negatively correlated with rumen microorganisms and fermentation, rate of organic matter degradation, microbial cell yield per unit organic matter fermentation and the ratio of transformation propionate to acetate in rumen fermentation and products [23]. Improved fiber digestibility and production seem to be one of advantages of supplementing yeast products which improve metabolic energy supply and explain the higher DMI [24] reported an improve in fiber digestion when added yeast products. The inclusion of yeast products to the diet benefits fiber digestion in ruminants through stimulating cellulolytic bacteria and preventing the decline in rumen pH caused by decreasing lactic acid production or increasing the bacterial utilization of lactic acid or both [25].The combining of Saccharomyces cerevisiae and Aspergillus oryzae exhibited significant increase in N-intake but without significant effect on N-excreted in faeces, as reported by [8]. So, the present study are in agreement with those obtained by [26] who reported that the microbial addition brought about less excretion in faecal nitrogen which lead to improvement in nitrogen balance. The present studies are in disagreement with their results concerning the urinary nitrogen. These results are in accordance with those obtained by [27] who found that the addition of biologically treated sugarcane bags did not Couse significant changes in blood serum level of total protein, albumin, globulin and consequently the ratio of alb/glob.Serum total protein is very important since it reflects the natural status of the animal and it has a positive correlation with dietary protein [28]. [29] Cleared that there was no significant effect of feeding lambs on wheat straw treated with Agaricus bisporus spawning on plasma total protein values. Mean values of plasma globulins tended to be insignificantly higher for lambs fed rations containing treated wheat straw then those feed the control ration. This may be due to that rations containing treated wheat straw improved albumin synthesis in the liver. Obtained results are accordance with those obtained by [30] and [31] who reported that biological treatments increased total of protein serum. They also reported that serum globulin was not affected by biological treatment. [32] Studied the values of blood serum total proteins and albumin for the animal fed rice straw treated with ZADR, fungus, ZADR + fungus and untreated. They found that blood serum total proteins and its fractions were within the normal values. So, these results approved that the microbiological treatment of poor quality roughages showed increase in their CP content digestibility when conditions are appropriate [33]. These results are in agreements with those of [34] who found no significant differences among examined treatments. Change in plasma urea would reflect changes in ruminal ammonia concentration. [27] found that the addition of biologically treated sugarcane bagasse did not cause significant changes in level of urea of blood serum. The activities of microorganisms in rumen increased the fermentation rat because of producing extracellular enzymes that lead to increase solubility of different nutrients specially proteins which increase in a farm of ruminal ammonia as reported by [33]. [29] pointed out that no significant differences were found among treatments in plasma serum aspartate aminotransferase (Ast) and alanine amino transfers (Alt) for lambs fed biologically treated rice straw compared with control ration. [30] And [31] reported that biological treatments increased aspartate aminotransferase. [32] Found that both aspartate aminotransferase (Ast) and alanine amino transfers (Alt) were unaffected with treatments but within the normal ranges. These results are also in line with those of [35] who reported that enzymatic treatment of wheat straw had no adverse effect on liver function. Also, [27] found that the addition of biologically treated sugarcane bagasse did not cause significant changes in GOT and GPT that in accordance with the present results. These results again are in harmony with those reported [36].
5. Conclusions
- From obtained results, it can be concluded that the microbiological pretreatment of agricultural by-products with reference to rice straw was very important to maximize significantly (P≤ 0.001) the nitrogen intake, urinary nitrogen and nitrogen digested which increase the use fullness of animal diets.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML