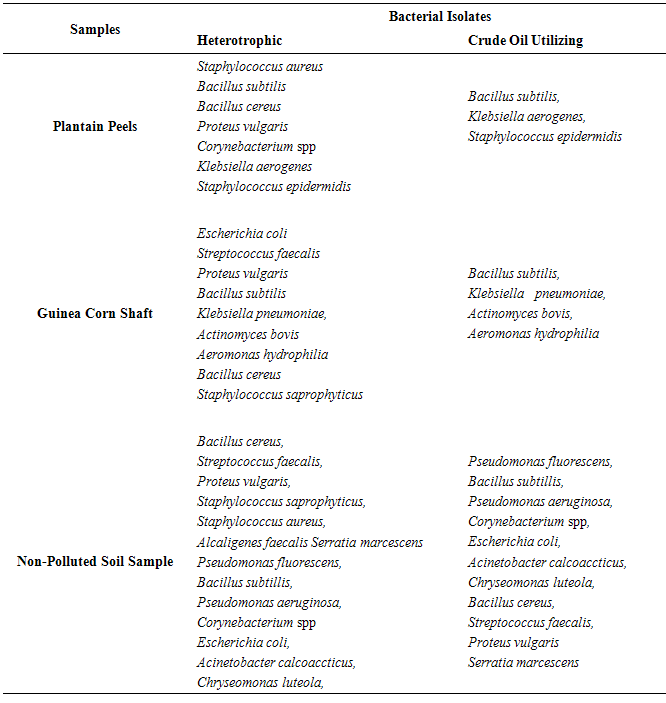
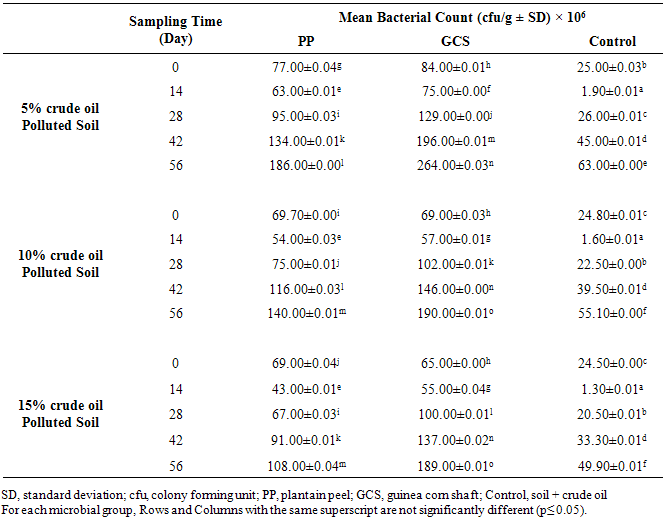
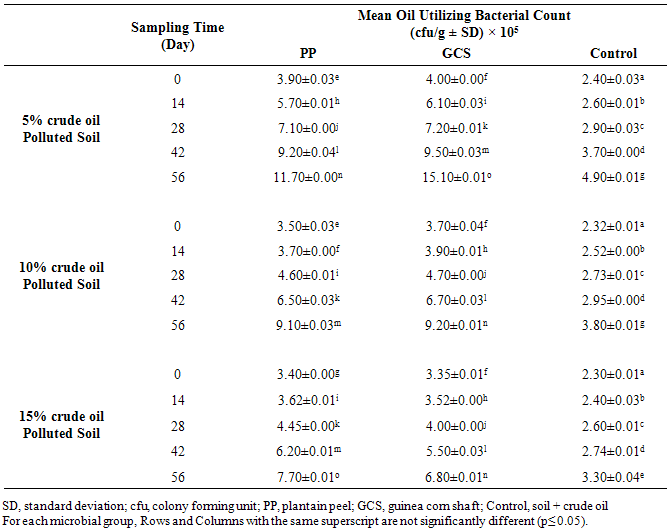
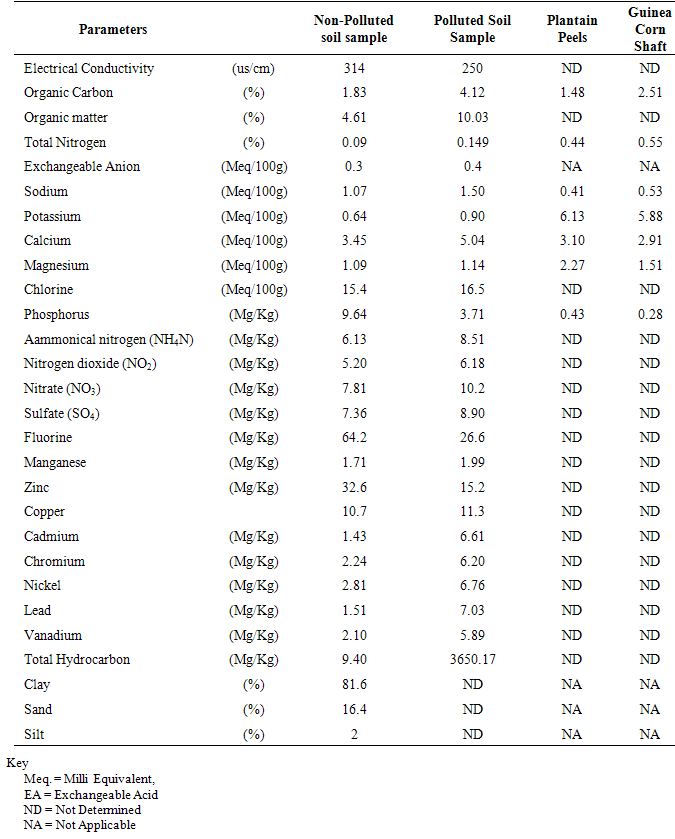
| [1] | Schafer, A.N., Snape, I. and Siciliano, S.D. (2009). Influence of liquid water and soil temperature on petroleum hydrocarbon toxicity in Antarctic soil. Environmental Toxicology and Chemistry, 28, 1409-1415. |
| [2] | Cunningham, C.J. and Philp, J.C. (2000). Comparison of bioaugmentation and biostimulation in ex-situ treatment of diesel contaminated soil. Land Contamination and Reclamation 8: 261–269. |
| [3] | Dorn, P.B., Vipond, J.P., Salanitro, T.E. and Wisniewski, H.L. (1998). Assessment of the acute toxicity of crude oil in soils using earthworms, microtoxic and plants. Chemosphere, 37, 845-860. |
| [4] | Ayotamuno, M.J., Kogbara, R.B. and Egwuenum, P.N. (2006). Comparism of corn and elephant grass in the phytoremediation of a petroleum hydrocarbon contaminated agricultural soil in Port-Harcourt, Nigeria. Journal of Agriculture and Environment, 4 (2&4), 218-222 |
| [5] | Walworth, J., Pond, A., Snape, I., Rayner, J., Ferguson, S., & Harvey, P. (2007). Nitrogen requirements for maximizing petroleum bioremediation in a sub-Antarctic soil. Cold Regions Science and Technology |
| [6] | Harley, J.P. and Prescott, L.M. (2004). Laboratory exercises in Microbiology 5th edn.McGraw Hill Publishers, New York 16-25 pp. |
| [7] | Agarry, S. E., Owabor, C. N., and Yusuf, R. O. (2010b). Bioremediation of soil artificially contaminated with petroleum hydrocarbon mixtures: Evaluation of the use of animal manure and chemical fertilizer. Bioremediation J. 14 (4): 189 - 195. |
| [8] | Akpe, A.R., Ekundayo, A.O. and Esumeh, F.I. (2014). Screening for crude oil degrading bacteria in liquid organic waste (Effluent samples). Pakistan Journal of Scientific and industrial Research. Series B: Biological Sciences 57(2): 86-91 |
| [9] | Semple, K. T., Dew, N. M., Doick, K. J., & Rhodes, A. H. (2006). Can mineralization be used to estimate microbial availability of organic contaminants in soil? Environmental Pollution, 140, 164–172. |
| [10] | Mark A.J. and Jeffrey, H.G. 1991. In-situ Comparison of bioremediation methods for a number of residual fuel spill in Lee County, Florida. Proceedings of the 1991 Oil Spill Conference, American Petroleum Institute, Washington D.C. pp. 533-530. |
| [11] | Vinas, P. Loopez-Garcia, I. Merino-Merono, B. Campillo, N. and Hermandez-Cordoba, M. (2005). Determination of selenium species in infant formulas and dietic supplements using liquid chromatography-hydride generation atomic fluorescence spectrometry. Analytica Chemica Acta 535 (1-2): 49-56 |
| [12] | Riffaldi, R., Levi-Minzi, R., Cardelli, R., Palumbo, S., & Saviozzi, A. (2006). Soil biological activities in monitoring the bioremediation of diesel oil-contaminated soil. Water, Air & Soil Pollution, 170, 3–15. |
| [13] | Agarry, S. E., Owabor, C. N. and Yusuf, R. O. (2010a). Studies on biodegradation of kerosene in soil under different bioremediation strategies. Bioremediation J. 14 (3): 135 – 141. |
| [14] | Abioye, O. P., Agamuthu, P and Abdul Aziz, A. R. (2012) Biodegradation of Used Motor Oil in Soil Using Organic Waste Amendments. Biotechnology Research International Volume 2012 (2012), Article ID 587041, 8 pages http://dx.doi.org/10.1155/2012/587041. |
| [15] | Agarry, S. E. and Jimoda, L. A. (2013). Application of carbon-nitrogen supplementation plant and animal sources in in-situ soil bioremediation of diesel oil experimental analysis and kinetic modeling. Journal of Environment and Earth Science 3 (7): 51-62. |
| [16] | Onuoha, S. C. (2013). Stimulated biodegradation of spent lubricating motor oil in soil amended with animal droppings. Journal of Natural Science Research 3 (12): 106-116. |
| [17] | Chijioke-Osuji, C. C., Ibegbulam-Njoku, P. N. and Belford, E. J. D. (2014). Biodegradation of crude oil polluted soil by composting with agricultural wastes and inorganic fertilizer. Journal of Natural Science Research 4 (6): 28-39. |
| [18] | Danjuma, B. Y., Abdulsalam, S. and sulaiman, A. D. I. (2012). Kinetic investigation of Escravos crude oil contaminated soil using natural stimulants of plantsources. Int. J. Emerging Trends in Eng. & Dev., 2 (5): 478-486. |
| [19] | Nyankanga, R. O., Onwonga, R. N., Wekesa, F. S., Nakimbugwe, D., Masinde, D. and Mugisha, J. (2012). Effect of inorganic and organic fertilizers on the performance and profitability of grain Amaranth (Amaranthus caudatus L.) in Western Kenya. J. Agric. Sci., 4 (1): 223―232. |
| [20] | Adesodun, J.K. and Mbagwu, J.S.C. (2008). Biodegradation of waste lubricating petroleum oil in a tropical alfisol as mediated by animal droppings. Bioresource Technology 99: 5659–5665. |
| [21] | Agarry, S. E. and Ogunleye, O. O. (2012). Box-behnken designs application to study enhanced bioremediation of soil artificially contaminated with spent engineoil using biostimulation strategy. Int. J. Energy and Environ. Eng. 3:31-34. |
| [22] | Mervat, S. M. (2009). Degradation of methomyl by the novel bacterial strain Stenotrophomonas maltophilia M1. Elect. J. Bacteriol. 12(4): 1-6. |
| [23] | Akpe, A.R., Ekundayo, A.O. and Esumeh F.I. (2013). Degradation of Crude oil by bacteria: A role for plasmid-borne genes. Global Journal of Scientific Frontier Research Biological Science 13 (Issue 6 Version 1.0): 21-26. Code 279999p. |
| [24] | Mills, A. L., Brenil, C. and Colwell, R. R. (1978). Enumeration of petroleum degrading marine and estuarine microorganisms by most probable number method. Can. J. of Microbiol. 24: 552-557. |
| [25] | Okpokwasili, G. C. and Amanchukwu, S. C. (1988). Petroleum hydrocarbon degradation by Candida spp Environ. Inter. 14: 243-247. |
| [26] | Gerhardt, P. (1994). Methods for General and molecular Bacteriology (ed.) ASM Press Washington DC. |
| [27] | Barrow, G.I. and Feltham, R.K.A. (eds.) (1986). Cowan and steel’s Manual for the Identification of Medical Bacteria. 3rd Edition. Cambridge university press 10- 68 pp. |
| [28] | Holt, J. G. (ed) (1994). Bergey’s Manual of Determinative Bacteriology 9th Edn. Williams and Wilkins Co., Baltimore |
| [29] | American Public Health Association (APHA) (1985). Standard Methods for the enumeration of water and waste. American Public Health Association, 15th edition Washington D.C. |
| [30] | Dhyan, S., Chhonkar, P. K. and Pandey, R. N. (1999). Soil, Plant and Water Analysis- A Method Manual. IARI, New Delhi. |
| [31] | Brady, N. C. and Weil, R. R. (1999). The Nature and Properties of Soils. 12th ed., Prentice Hall Publishers London. Pp 740. |
| [32] | Olsen, D. W. and Sommers, L. E. (1982). Determination of total organic carbon. In: Methods of Soil Analysis Part 2 (Chemical and Microbiological Properties) Agronomy Monograph No 9 Pp539-560. |
| [33] | Van Hamme, J. D., Singh, A and O. P. Ward, (2003) Recent advances in petroleum microbiology. Microbiology and Molecular Biology Reviews, 67 (4): 503–549. |
| [34] | Foght, J. M. and Westlake, D. W .S. (1987). Biodegradation of hydrocarbon in fresh waters, In: Vandermuelen, J. H. Hrudy, S. E. (eds.) Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. Pergamon Press, New York. Pp. 252-263. |
| [35] | Esumeh, F. I., Akpe, A. R., Eguagie, O. E. (2009). Crude oil Degrading Capabilities of bacterial isolates from pawpaw (Carica papaya) and sweet orange (Citrus sinensis). A role for plasmid mediated gene. Proceedings of the 1st International Conference, Workshop and exhibition on Biotechnologies for Improved Production of Oil and Gas in the Gulf of Guinea, held in Abuja, Nigeria 1. April 1-3. 2009. BIPOG3-4-34. Pp. 1-7. |
| [36] | Jørgensen, K. S., Puustinen, J., & Suortti, A. M. (2000). Bioremediation of petroleum hydrocarbon-contaminated soil by composting in biopiles. Environmental Pollution,107 (2): 245–254. |
| [37] | Vahaboglon, H., Dodanli, S. and Ozturk, R. (1996). Characterization of multiple antibiotic resistant Salmonella typhimurium strains, molecular epidemiology of PCR-1-producing isolates and evidence for nosocomial plasmid exchange by a clone. J. Clin Microbiol. 34: 2942 -2946. |
| [38] | Walkley, A. and Black, I. A. (1934). An examination of the Degtjarf method for determining Soil organic matter and a proposed modification of the chronic acid titration Method. Soil Science 37: 29-38. |
| [39] | Ijah, U. J. J. and Antai, S. P. (2003). The potential use of chicken-drop microorganisms for oil spill remediation. Environmentalist 23 (1): 89–95. |
| [40] | Joo, H. S., Shoda, M. and Phae, C. G. (2007). Degradation of diesel oil in soil using a food waste composting process. Biodegradation 18 (5): 597–605 |
| [41] | Abioye, O. P., Alonge, O. A. and Ijah, U. J. J. (2009). Biodegradation of Crude Oil in Soil Amended with Melon Shell. AU J. T. 13(1): 34-38. |
| [42] | Mbah, C. N., Nwite, J. N. and Nweke, I. A. (2009) Ameriolation of spent oil contaminated ultisol with organic wastes and its effect on soil properties and maize (Zea mays L) yield. World. J. Agric. Sci. 5(2), 163-168. |
| [43] | Akoachere, J.T.K., Akenji, T.N., Yongabi, F.N., Nkwelang, G and Ndip, R.N (2008) Lubricating oildegrading bacteria in soils from filling stations and automachanic workshops in Buea, Cameroon: occurrence and characteristics of isolates. African. J. Biotechnol. 7, 1700-1706. |
| [44] | Odokuma, L. O. and Ibor, M. N. (2002). Nitrogen fixing bacteria enhanced bioremediation of a crude oil polluted soil. Glob. J. Pure Appl. Sci.8(4):455-468. |
| [45] | Kim, S.J., Choi, D.H., Sim, D.S. and Oh, Y.S. (2005) “Evaluation of bioremediation effectiveness on crude oil-contaminated sand,” Chemosphere 59(6): 845–852. |
| [46] | Okoh, I. O. (2006) “Biodegradation alternative in the cleanup of petroleum hydrocarbon pollutants,” Biotechnology and Molecular Biology 2: 38–50. |
| [47] | Dibble, J.T. and Bartha, R. (1979). Rehabilitation of oil-Inundated agricultural land: A case history. Soil Science 128 (1): 56-60. |
| [48] | Lee, K. and Trembley, G.H. (1993). Bioremediation: Application of slow-release fertilizers on slow energy shorelines. Proceedings of the 1993 Oil Spill Conference, American Petroleum Institute, Washington, D.C. 449-454 pp. |
| [49] | Tanee, F. B. G. and Kinako, P. D. S. (2008). Comparative Studies of Biostimulation and phytoremediation in the mitigation of crude oil toxicity in tropical soil. J. Appl. Sci. Environ. Manage. 12(2): 143 – 147. |
| [50] | Obasi, N. A., Eberechukwu, E., Anyanwu, D. I. and U. C. Okorie (2013). Effects of organic manures on the physicochemical properties of crude oil polluted soils. African Journal of Biochemistry Research 7(6): 67-75. |

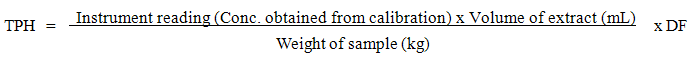 Where TPH = Total petroleum hydrocarbon
Where TPH = Total petroleum hydrocarbon  DF = Dilution factor
DF = Dilution factor  Conc. = Concentration
Conc. = Concentration
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML