-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2015; 5(1): 11-22
doi:10.5923/j.microbiology.20150501.02
Plasmid Curing and Its Effect on the Growth and Physiological Characteristics of Lactobacillus plantarum Isolated from Fermented Cereals
Adeyemo S. M.1, Onilude A. A.2
1Department of Microbiology, Obafemi Awolowo University, Ile –Ife, Nigeria
2Department of Microbiology, University of Ibadan, Ibadan, Nigeria
Correspondence to: Adeyemo S. M., Department of Microbiology, Obafemi Awolowo University, Ile –Ife, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Plasmids play important roles in the lives of organisms that have them and have proved invaluable to microbiologists and molecular geneticists in constructs and transferring new genetic combinations cloning gene. Not all organisms and lactic acid bacteria have plasmids. They confer a selective and important advantage on the organisms that possess them such as L. plantarum. Plasmid extraction and electrophoresis of the DNA was carried out on 0.8% agarose gel in a 0.5X concentration of Tris-Borate- EDTA (TBE) buffer. Plasmid DNA bands were viewed by fluorescence of bound ethidium bromide under a short wave ultraviolet light trans-illuminator and the photographs taken. Plasmids curing were done by the use of acridine orange after the growth of the organisms. Physiological studies were done by monitoring the growth through increased turbidity of the isolates at different environmental conditions spectrophotometrically. Forty L. plantarum were screened, only eleven of them possess plasmids; Out of which only three were chosen for further work based on the abundant production of plasmids. These were subjected to different conditions with the cured and uncured isolates. Plasmid curing showed a significant negative effect on growth, physiological characteristics and colony morphology of the L. plantarum.
Keywords: Plasmids curing, L. plantarum, Electrophoresis, Profiling, Growth, Physiological studies
Cite this paper: Adeyemo S. M., Onilude A. A., Plasmid Curing and Its Effect on the Growth and Physiological Characteristics of Lactobacillus plantarum Isolated from Fermented Cereals, Journal of Microbiology Research, Vol. 5 No. 1, 2015, pp. 11-22. doi: 10.5923/j.microbiology.20150501.02.
Article Outline
1. Introduction
- Plasmids are extra chromosomal DNA molecules. They are circular DNA molecules that replicate independently of the bacterial chromosome (Wang et al., 2011). Plasmids are small, circular molecules of DNA containing genetic information. They contain about two percent of the genetic information of a cell and are separate from chromosomes. They are essential for the bacterium in that they may give it a selective advantage. Some plasmids determine the production of proteins that can kill other bacteria; others make bacteria resistant to antibiotics. Plasmids are extremely valuable tools in the fields of molecular biology and genetics, specifically in the area of genetic engineering (Bruce et al., 2002; Bulut et al., 2005).In general, bacterial plasmids can be classified into two groups on the basis of the number of genes and functions they carry. The larger plasmids are Deoxyribonucleic acid (DNA) molecules of around 100 kilobase (kb) pairs, which is sufficient to code for approximately 100 genes. There are usually a small number of copies of these plasmids per host chromosome, so that their replication must be precisely coordinated with the cell division cycle. The plasmids in the second group are smaller in size, about 6–10kb. These plasmids may harbor 6–10 genes and are usually present in multiple copies (10–20 per chromosome) (Bruce et al., 2002; Prescott et al., 2009; Wang et al., 2011). Both linear and circular plasmids have been documented, but most known plasmids are either circular or linear which possess special structure or sequence at the ends to prevent their degradation and to permit their replication. They have few genes generally less than 30. Their genetic information is not essential to the host and all the cells that lack them usually function normally. However, many plasmids carry genes that confer a selective advantage to their hosts in certain environment (De Magalhaes et al., 2008; Mohania et al., 2008).Plasmids are able to replicate autonomously. Single copy plasmids produce only one copy per host cell, some are able to integrate into the chromosome and are thus replicated with the chromosomes. Such plasmids are called Episomes. Plasmids are inherited stably during cell division, but are not always equally apportioned into daughter cells and sometimes are lost (Bulut et al., 2005; Mohammed et al., 2008). They carry genes that confer a selective advantage on their host, such as resistance to heavy metals or resistance to naturally made antibiotics carried by other organisms. Alternatively, they may produce antibiotics that help the host to compete for food or space. For instance, genes produced by a plasmid will allow its host bacteria to grow even in the presence of competing bacteria or fungi that produce these antibiotics (De Magalhaes et al., 2008; Mohania et al., 2008).Plasmids are also useful tools in research and biotechnology. Because of their ability to move genes from cell to cell; plasmids have become versatile tools for research and biotechnology. In the laboratory, researchers use plasmids to carry marker genes, allowing them to trace the plasmid's inheritance across host cells. Transferred or "cloned" genes are used to produce a variety of important medical, agricultural, or environmental products that can be economically used by humans (Bruce et al., 2002; Wang et al., 2011).There are different types of plasmids namely Conjugate plasmids, this confer hair like structures called pilli and can transfer copies of themselves to other bacteria during conjugation. F-factor: it is responsible for the formation of fertility factor. Resistance factor: R-factors: They confer antibiotics resistance on cells that contain them. R plasmids carry genes encoding resistance to antibiotics. Bacteria encoding plasmids: are bacteria proteins that encode other bacteria. All bacterial genes are located on the plasmids. Virulence plasmids: Encode factors that make the host more pathogenic. They confer pathogenicity on a host organism by the production of toxins or other virulence factors. Metabolic plasmids / Degradative or catabolic plasmids: They carry the genes required to break down complex sugars into simple sugars. They also allow a host bacterium to metabolize normally undegradable or difficult compounds such as various pesticides and chemicals (Alcamo et al., 2000; De Magalhaes et al., 2008; Mohania et al., 2008; Wang et al., 2011).Plasmid Curing: The loss of plasmid is called curing. It can occur spontaneously or be induced by treatments that inhibit plasmid replication but not host cell reproduction. Some commonly used curing treatments are acridine mutagens, ion and ionizing radiation, thyme starvation, antibiotics and growth above optimum temperature, pH or extreme environmental conditions (Ogier et al., 2002). Because of their ability to move genes from cell to cell, plasmids have become versatile tools for both research and biotechnology. In the laboratory, researchers use plasmids to carry marker genes, allowing them to trace the plasmid's inheritance across host cells. Transferred or "cloned" genes are used to produce a variety of important medical, agricultural, or environmental products that can be economically used by humans (Alcamo et al., 2000; Bulut et al., 2005).Lactic acid bacteria are a heterogeneous group of bacteria that are generally regarded as safe (GRAS). The use of these organisms in food products dates back to ancient time, and they are used mainly because of their contribution to flavour, aroma and increased shelf life of fermented products (Adeyemo, 2012).Various members of this group are used commercially as starter cultures in the manufacture of foods including dairy Lactobacillus plantarum has been used for the preservation of food for increased shelf life and flavour to get the desired aroma in food (Adeyemo and Onilude, 2014a). Fermentation with cultures containing LAB is able to produce healthy, safe, high quality and nutritious beneficial food products such as fermented milk, meat, vegetables, grains, cereals, legumes, meat, beverages, etc. The organisms produce lactic acid which has a way of preserving such fermented foods and also improve the flavour, texture and nutritional compounds of such foods through the metabolic activities of LAB during fermentation. Also, the metabolism and physiology of LAB is used in different biotechnological processes in industries to formulate LAB starters with useful metabolic activities and capabilities so as to ensure a wide range of quality fermented products with consistent characteristics (Adeyemo and Onilude, 2014b). Being used as ‘probiotics’ and starter culture in many food industries and in fermentation technology, there is therefore the need to preserve the various metabolic activities of this group of organisms such as the production of plasmids.
2. Materials and Methods
2.1. Plasmid Analysis
2.1.1. Plasmid Isolation and Profiling
- Plasmid extraction was carried out using the method described by Brown (2000). Pure isolates were inoculated on MRS broth and incubated. The grown cells were harvested and suspended in 200µl of solution A (100 mM glucose-50 mMTris hydrochloride (pH 8)-10 mM EDTA) containing 10 mg of lysozyme per ml and 10μg/ml mutanolysin and incubated for 30 min at 37°C in an incubator. 400µl of freshly prepared 1% sodium dodecyl sulfate in 0.2 N NaOH was added and the samples were mixed by inverting the Eppendorf tubes. 600µl of a 15% potassium acetate solution (pH 4.8) was added and the samples were mixed by vortexing. After incubating on ice for 5 minutes, the debris was removed by a 5-minute centrifugation in a centrifuge (model 5415R; Eppendorf). The supernatant was removed and extracted once with a phenol-chloroform mixture (1:1) and precipitated with an equal volume of isopropanol. The plasmid DNA was then dissolved in 100µl of TE buffer.Electrophoresis of the DNA was carried out on a 0.8% agarose gel in a 0.5X concentration of Tris-Borate-EDTA (TBE) buffer. Agarose gel was prepared by boiling 0.8g of agarose powder in 100mls of 0.5X TBE buffer. After boiling, the solution was allowed to cool and 10µl of ethidium bromide was added to the cooled agarose solution. This was poured into a casting tray with a comb placed across its rim to form wells. The gel was allowed to set for 30 minutes and the comb was removed. 20µl of the plasmid DNA samples were then loaded into the wells after mixing with 2µl of bromophenol blue. A DNA molecular weight marker was also loaded into one of the wells. The gel was there after electrophoresed in a horizontal tank at a constant voltage of 60V for about 1 hour 30 minutes.After electrophoresis, plasmid DNA bands were viewed by fluorescence of bound ethidium bromide under a short wave ultraviolet light trans-illuminator and the photographs were taken using a Samsung 12.2 Mega Pixel, Germany digital camera.
2.1.2. Plasmid Curing
- The plasmids were cured by treatment with acridine orange according to the method of Brown (2000). Nutrient broth was prepared and supplemented with 0.1mg/ml acridine orange. 20µl of overnight culture of the bacteria was subcultured into 5 mls of the nutrient broth containing acridine orange. The samples were then incubated at 37°C for 72hours. After 72 hours incubation, the isolates were subcultured onto Mueller Hinton agar and plasmid extraction was repeated on some of the organisms to verify if the plasmid were successfully cured.
2.1.3. Growth at Different Temperature Ranges Using Uncured and Cured L. plantarum
- The test was done to determine the best temperature and pH for the growth and metabolism of the isolates as indicated by increased turbidity. MRS broth was prepared and dispensed into series of screw-capped bottles and sterilized. It was allowed to cool and the test organisms inoculated into it. These were incubated at different temperatures 20℃, 30℃, 40℃, 50℃, 60℃ and 70℃ for 72hrs after which Cecil 2031 spectrophotometer was used to detect increase in turbidity. The Effect of plasmid curing on the growth of the isolates was also studied. Un-inoculated tubes served as control (Adeyemo, 2012; Sneath et al., 2009; Olaoye et al., 2008).
2.1.4. Growth at Different pH Ranges Using Uncured and Cured L. plantarum
- This test was done to determine the best pH for the growth of the isolates as indicated by the increased turbidity .MRS broth was prepared and the pH was adjusted using 0.1m phosphate buffer to 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5 and 7.0. It was then dispensed into screw capped bottles and sterilized in the autoclave at 121℃ for 15mins. After cooling, the various test isolates were standardized using McFarland standard to 3.1 x 10 3 cfu/ml with optical density adjusted to 0.5. With a sterile pipette, 1ml of the standardized was isolates inoculated into it and incubated at 30℃ for 48hrs. Growth was detected by increased turbidity using Cecil 2031 spectrophotometer. The Effect of plasmid curing on the growth of the isolates was also studied .Un-inoculated tube served as control. (Adeyemo, 2012; Sneath et al., 2009; Olaoye et al., 2008).
2.1.5. Growth at Different Anion Concentration Using Uncured and Cured L. plantarum
- This test was done to detect the best concentration of the anion that favour growth and metabolism of the isolates MRS broth was prepared and varying concentration of anions in form of its salt (mM concentration) was used .The anions used were Triammonium citrate, Magnesium chloride and Sodium chloride. The concentration used was within the range of 0.1-2.0mg/ml, 10ml each was dispensed into screw capped bottles. This was sterilized, allowed to cool and the organisms inoculated into it. It was incubated at 30℃ for 48hrs. Growth was detected by increased turbidity using Cecil 2031 spectrophotometer. The Effect of plasmid curing on the growth of the isolates was also studied. Un-inoculated tube served as control. (Adeyemo, 2012; Sneath et al., 2009; Olaoye et al., 2008).
2.1.6. Growth of L. plantarum at Different Cation Concentration
- This test was done to detect the best concentration of cation that favours the growth and metabolism of the various isolates. MRS broth was prepared and varying concentration of cations in form of its sugar (mM concentration) was used. The cations used were magnesium sulphate, ferrous sulphate and zinc sulphate. The concentration used was within the range 0.1-1.5mg/ml. 10ml each was dispensed into screw-capped bottles. This was sterilized, allowed to cool and the test organisms inoculated into the bottles. It was then incubated at 30℃ for 48hrs after which growth was observed. Growth was detected by increased turbidity using Cecil 2031 Spectrophotometer. The Effect of plasmid curing on the growth of the isolates was also studied Un-inoculated tube served as control. (Adeyemo, 2012; Sneath et al., 2009; Olaoye et al., 2008).
2.1.7. Growth of L. plantarum at Different Concentration of Carbon Sources
- This test was done to detect the best concentration of the sugars that favour growth and metabolism of the isolates. MRS broth was prepared and varying concentration of carbon sources (mM concentration) - Glucose, Lactose and Raffinose were used. The concentration used was within the range of 0.1-2.0mg/ml and 10ml each was dispensed into screw-capped bottles. This was sterilized by membrane filtration, allowed to cool and the organisms inoculated into it. Uninoculated tubes with the carbon sources served as control. It was incubated at 30℃ for 48hrs. Growth was detected by increased turbidity using Cecil 2031 Spectrophotometer. The Effect of plasmid curing on the growth of the isolates was also studied (Adeyemo, 2012; Sneath et al., 2009; Olaoye et al., 2008).
2.1.8. Colony Morphology of Cured and Uncured L. plantarum Organisms on MRS Plate
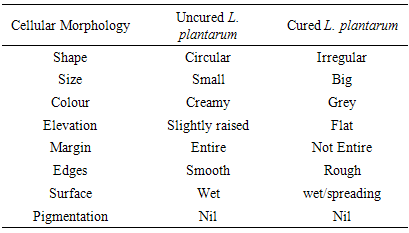
- Macroscopic observation of the different colonies as they appeared on MRS agar after incubation was done. The colour, shape, smell, appearance, size, elevation, edges and degree of growth, production of pigment, surface and margin were also noted and recorded. Pure cultures were examined microscopically using high power objective with immersion oil. The Effect of plasmid curing on the growth of the isolates was also studied (Adeyemo, 2012; Brown, 2000; Sneath et al., 2009).
2.1.9. Statistical Analysis
- The stastical analyses carried out include Mean, Standard Error, Analysis of Variance, Duncan Multiple Range of Variables using SARS Analytical Package.
3. Results and Discussion
- Figures 1 shows the plasmid picture before the curing while Figure 2 shows the plasmid picture after the curing of the nine L. plantarum isolates. It could be observed that it was the sample labeled M which is the marker that only showed the band and the molecular weight of the marker. The other ten isolates bands did not show the plasmid, this is because it has been cured/ destroyed. Once the plasmid has been cured, the bands will no longer show in the gel.
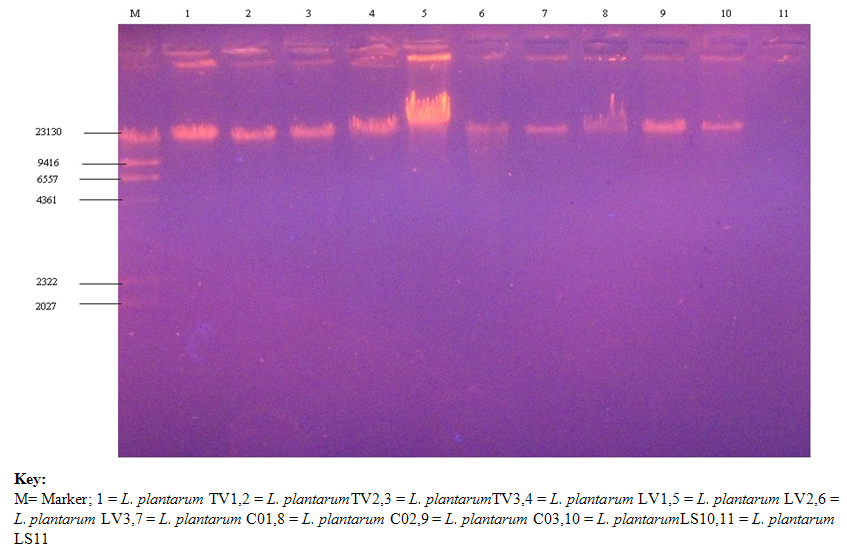 | Figure 1. Plasmid Picture of selected nine L. plantarum isolates before Curing |
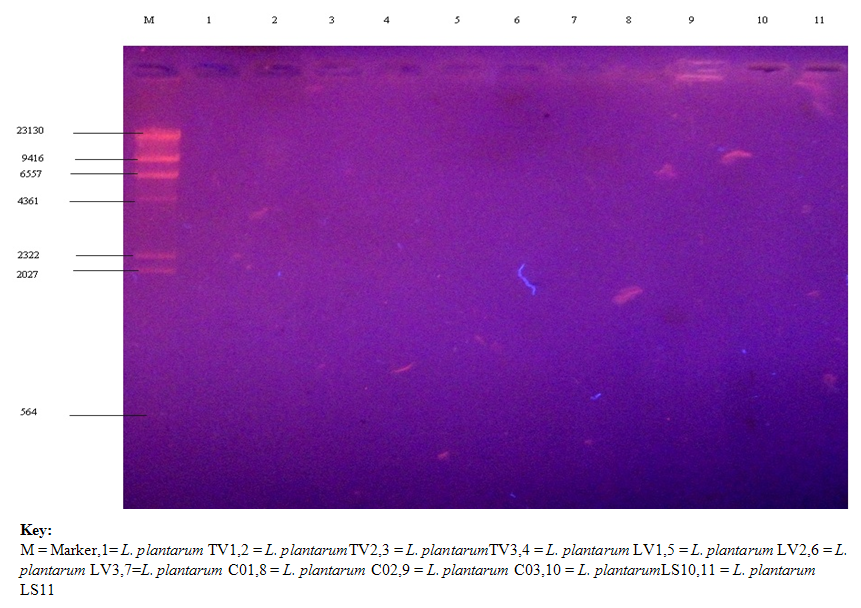 | Figure 2. Plasmid Profile of selected nine L. plantarum isolates after Curing |
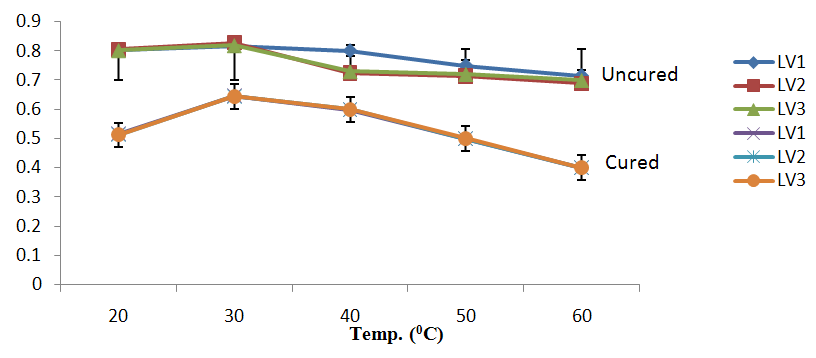 | Figure 3a. Growth of L. plantarum Isolate at Different Temperature ranges using Raffinose as Carbon Source |
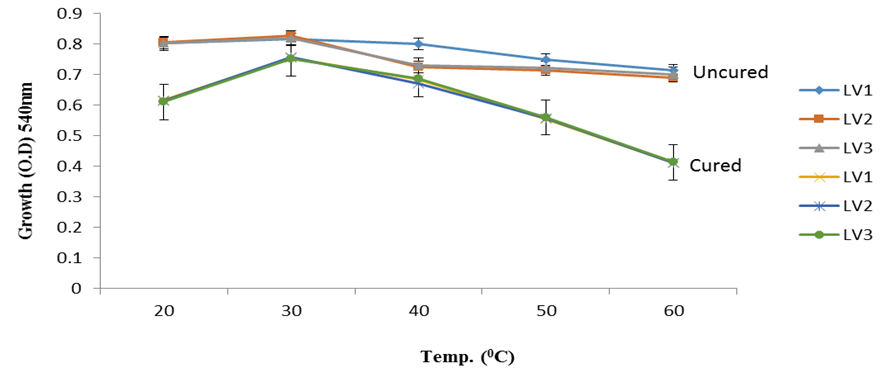 | Figure 3b. Growth of L. plantarum Isolate at Different Temperature ranges Using Lactose as Carbon Source |
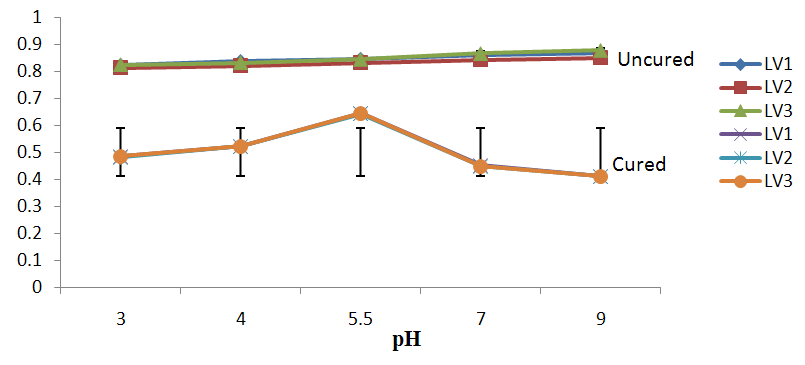 | Figure 4a. Growth of L. plantarum Isolate at Different pH ranges Using Lactose as Carbon Source |
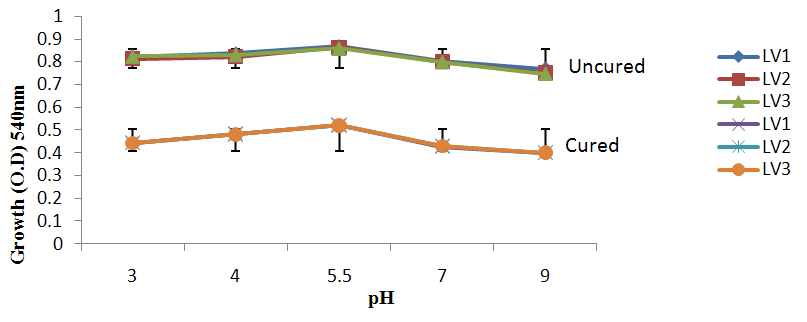 | Figure 4b. Growth of L. plantarum Isolate at Different pH ranges Using Raffinose as Carbon Source |
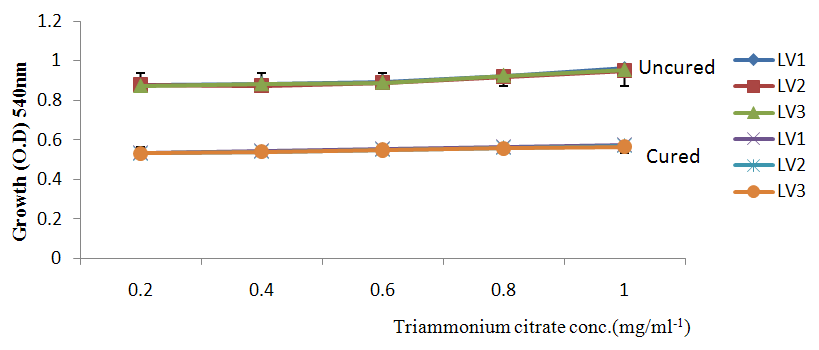 | Figure 5a. Growth of L. plantarum Isolate in Different Concentration of Triammonium Citrate Using Glucose as Carbon Source |
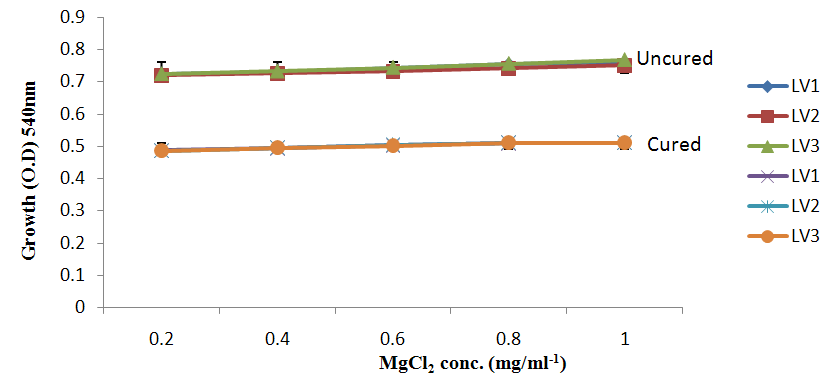 | Figure 5b. Growth of L. plantarum Isolate in Different Concentration of anion |
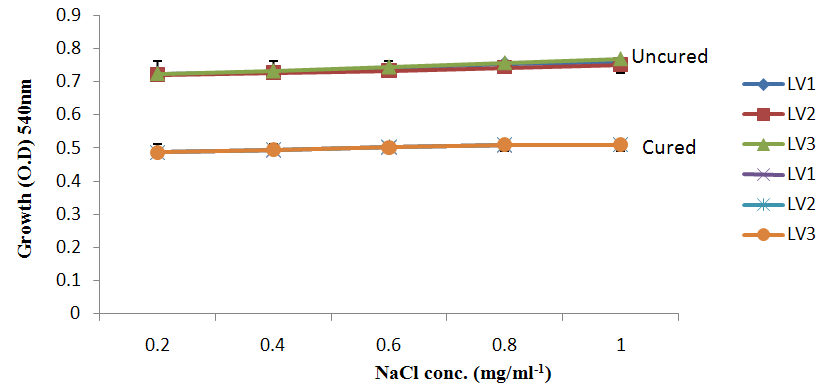 | Figure 5c. Growth of L. plantarum Isolate in Different Concentration of anion |
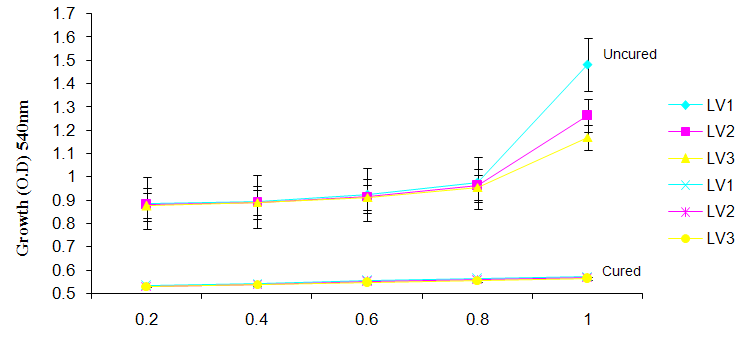 | Figure 6a. Growth of L. plantarum Isolate in Different Concentration of (MgS04) cation |
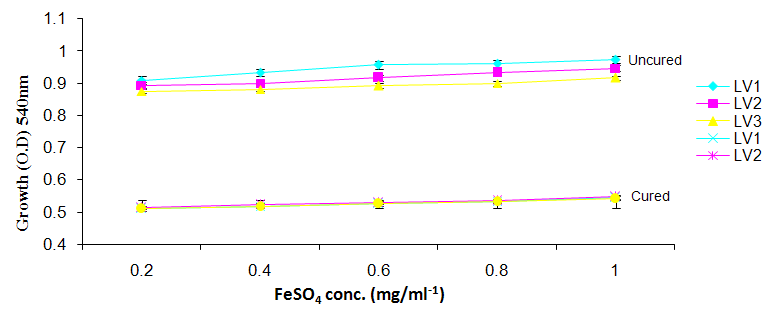 | Figure 6b. Growth of L. plantarum Isolates in Different Concentration of FeS04 |
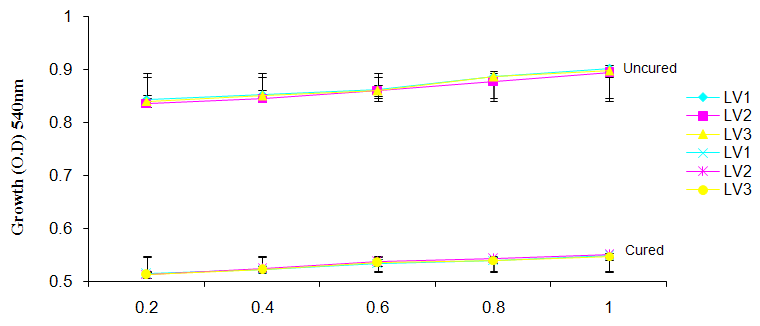 | Figure 6c. Growth of L. plantarum Isolate in Different Concentration of ZnS04 |
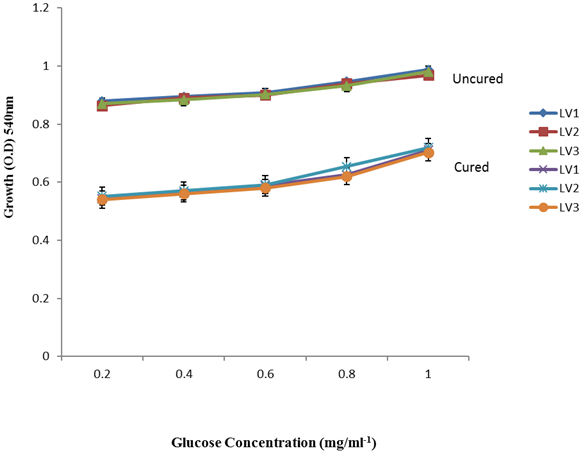 | Figure 7. Growth of L. plantarum Isolates in Different Concentration of Glucose |
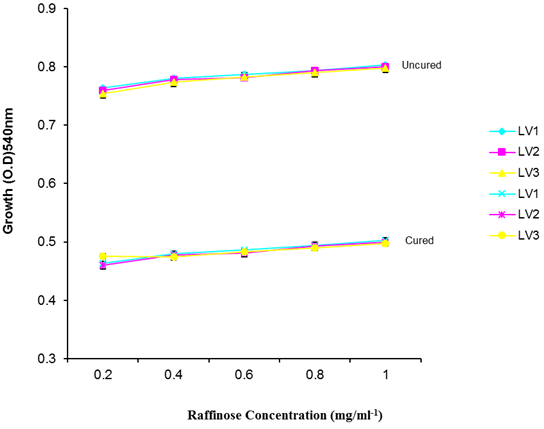 | Figure 8. Growth of L. plantarum Isolates in Different Concentration of Raffinose |
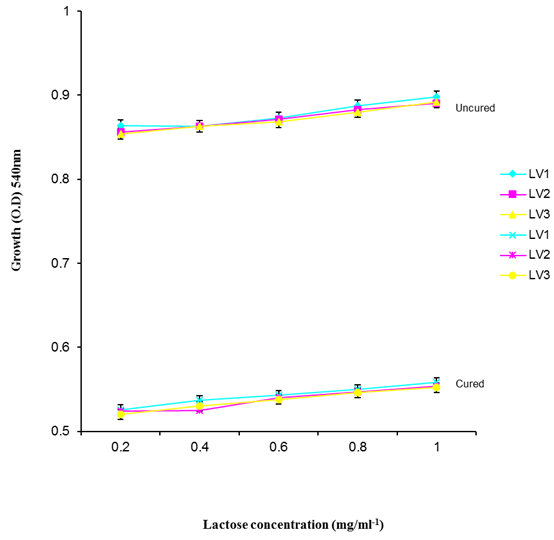 | Figure 9. Growth of L. plantarum Isolates in different concentration of Lactose |
|
4. Results and Discussion
- It was observed clearly in this study that the growth, the physiological and the colony characteristics were affected by plasmid curing. The various physiological studies show that L. plantarum was able to metabolize different sugars as sources of carbon for growth. Utilization of glucose was the highest; followed by lactose and raffinose the least. This is in agreement with the work of Adeyemo (2012) reported that all LAB ferments glucose with or without the production of gas. Bloom (2006) also stated that L. plantarum ferments different sugars at varying degrees with glucose taking a lead. The various LAB were able to utilize different carbon sources for growth – glucose, lactose and raffinose. This observation agrees with the work of Wakil and Onilude (2009) which stated that LAB are able to utilize different sugars as their main source of carbon to produce energy which they use for growth and metabolite production. It was also observed that different sugars are important component of microbial nutrition which are used for growth, energy and metabolite production. The organisms metabolize glucose and lactose better than raffinose, but the ability of L. plantarum to metabolize raffinose is a desirable attribute in microbial nutrition and metabolism Adeyemo (2012) and Adeyemo and Onilude (2014a&b). Others also reported that LAB metabolize raffinose through the Embden Meyerhof Pathway. This is in agreement with the work of Bloom (2006) and Adeyemo and Onilude (2014a) that stated that LAB ferment the oligosaccharide raffinose in a very weak or slow manner. Also, bacteria lose their plasmids which leads to metabolite inconsistencies because some sugar fermentative capacities are plasmid encoded. Moreover, physiological studies involving the use of cured and uncured organisms in sugar metabolism were monitored in relation to raffinose metabolism. The study showed that there was a drastic reduction in the growth and utilization of anions and cations of the organisms being monitored. Plasmid curing affected the ability of these organisms to grow in these various environmental conditions. This observation is in agreement with the work of Ben et al., (2006) who stated that LAB grows at optimum temperature and conditions of growth. At, adverse environmental conditions, which is thus implicated in different methods used for curing, the organisms did not grow well, as a result, they cannot produce metabolites. This is in accordance with the opinions of Messens and De Vuyst (2002). Furthermore, Ben et al., (2006) reported that at a very high temperature above 60℃, LAB will not grow nor produce enzymes. This was observed in the physiological studies on the organisms isolated from this work.This work also showed that plasmid curing reduced the ability of L. plantarum to metabolize raffinose and other sugars used in this work. When the cured isolate were used in the various physiological processes, the growth of the organisms were rather very slow. According to Adeyemo (2012) the metabolism and utilization of raffinose by LAB is a plasmid related trait. The colony morphology of the organisms was also affected by curing. This is in agreement with the work of Alcamo et al. (2000); De Magalhaes et al., (2008) and Mahania et al., (2008) that reported that the ability of LAB to ferment sugars which are commonly found in plants is a selective advantage. The presence of plasmids however governs the fermentation of carbohydrates in LAB. This is of utmost importance in the food fermentation industry.Since LAB are fastidious organisms, the media that they grow in are usually supplemented with anions and cations to meet their complex nutritional requirements. According to Dugas (2004), they require accessory growth factors that can meet their nutritional demand and biosynthetic capabilities. MgS04 was preferred by the organisms than FeS04 and ZnS04 because it meets their demand for minerals salt and growth requirement Jahns et al. (2005). Also, the anion triammonium citrate was preferred by the organisms to NaCl and MgCl2 from the result obtained in the physiological studies of the isolates. Jahns et al, (2005) stated that the growth of LAB isolates in the presence of different inorganic salt form complexes that is produced by the organisms. The various mineral salts or anions and cations added to the medium of growth improved the performance of the organisms. This made them performed well when their physiology and growth were monitored and they were compared with the ones in which the mineral salts were not added. Optimization of the minimal basal requirement of growth showed that when the medium of growth are supplemented with accessory growth factors, L. plantarum performed better. According to Ben et al. (2006) and Dugas (2004), an appropriate culturing medium must however contain a carbon source, nitrogen source, anions, cations, and mineral salts. As carbon sources in the growth of L. plantarum, glucose, lactose, raffinose and other sugars can be used, also glycerol and sodium acetate can be used. As nitrogen sources in the growth of L. plantarum, inorganic and organic nitrogen compounds, such as meat extract, yeast extract, peptone, amino acids, casein hydrolysate, soybean flour and ammonium salts can be used. The pH range for culturing is from is preferably from 5.0 to 5.5, the temperature is comprised between 30℃ and 40℃.Ben et al. (2006) and Gomez et al. (2008) also reported that the activities of microorganisms are enhanced by the presence of metal ions like Mg2+ and Na+ in the growth medium. Microorganisms require free metals for their activity such as the ones stated above, it is therefore necessary to ensure that the metals are in sufficient amount in the medium of growth of the isolates as earlier reported by many authors. Despite all these, the cured L. plantarum showed a reduction in growth, this is because plasmid curing has affected the ability of the organisms to utilize the metals and the ability to metabolize the growth factors that are required by the organisms. There was however, a sharp reduction in their physiological characteristics and growth as a result of this. As stated earlier by Brown (2000) the selective advantage that is conferred on the organisms by plasmid presence is usually lost during plasmid curing. Plasmids are involved in the replication of organisms; this is why their absence in an organism affects its growth Mohammed et al. (2011).The optimum temperature and pH required for the growth of LAB isolates as stated by Olaoye and Onilude (2009) and Adeyemo (2012) is at temperature of 30℃ and a pH of 5.5. The uncured organisms L. plantarum grew well at this temperature and pH in the presence of raffinose. A lower temperature or pH did not favour the growth of the organisms. At optimum temperature and pH, uncured L. plantarum performed at their best, while there was reduction in growth at very high/low temperature and pH which affected the production of metabolites by the isolates. This was also reported in the work of Messens and De Vuyst (2002) who observed that the growth of LAB are usually affected by extreme environmental conditions. Plasmid curing however, affected the ability of the organism to grow well and utilize the various carbon sources for growth even at the optimum environmental conditions, this is in agreement with the work of Bruce et al. (2002) that opined that plasmids are easily lost at extreme environmental conditions and as a result the organisms will not grow well or perform at their best.
5. Conclusions
- According to Mahania et al. (2008), who reported that, bacterial isolates do not all their genes and plasmids at the same time because gene expression is related to environmental conditions. Also, Ben et al. (2006) reported that most metabolites produced by LAB are sometimes affected and destroyed by heat treatment because they are proteins, hence the destruction of plasmids of LAB when they are subjected to extreme environmental condition. Also, the report of this study also agrees with that of De Magalhaes et al. (2008) who confirmed that bacteria lose their plasmids which leads to metabolite inconsistencies because some fermentative capacities are plasmid encoded. LAB is used in different biotechnological processes in industries to formulate LAB starters with useful metabolic activities and capabilities so as to ensure a wide range of quality fermented products with consistent characteristics. Being used as probiotics and starter culture in many food industries and in fermentation technology, there is therefore the need to preserve the various metabolic activities of this group of organisms such as the production of plasmids.As demonstrated in this study, the growth of L. plantarum and the various physiological processes is plasmid related. L. plantarum lose their plasmids easily as a result of handling e.g. the use of heat in soaking cereals for fermentation, poor handling conditions, and the use of chemicals during storage or preservation of cereals, poor storage condition of gruels and other environmental factors make them to lose their plasmids easily. Efforts should therefore be directed towards preserving the various qualities that are found in this organism.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML