-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(6): 193-200
doi:10.5923/j.microbiology.20140406.02
HPLC Protocol for Identification of Mycobacterium spp. from Clinical Samples of Human and Veterinary
Leone Vinícius Furlanetto1, Carlos Adam Conte-Júnior1, 2, Eduardo Eustáquio de Souza Figueiredo3, Rafael Silva Duarte1, Walter Lilenbaum2, Joab Trajano Silva1, Vânia Margaret Flosi Paschoalin1
1Universidade Federal do Rio de Janeiro, Rio de Janeiro/RJ, Brazil
2Universidade Federal Fluminense, Niterói/RJ, Brazil
3Universidade Federal de Mato Grosso, Cuiabá/MT, Brazil
Correspondence to: Carlos Adam Conte-Júnior, Universidade Federal do Rio de Janeiro, Rio de Janeiro/RJ, Brazil.
| Email: | 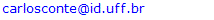 |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract HPLC is considered one of the most reliable and cost-effective tools for the rapid identification of Mycobacterium spp. isolated in culture. In this study, the CDC protocol developed in the early 1990s and originally designed for a short column was transferred to a 33% longer column with similar stationary-phase properties. Mycolic acids from cell walls of 35 different Mycobacterium reference strains were saponified, extracted, derivatised, analysed and used to compose a library for successful identification of clinical samples by the adapted-HPLC protocol. The identification of mycobacteria was based on comparison of the relative retention times (RRT) of the chromatogram patterns with those obtained from reference strains and with those available in external databases. Although an internal standard was not used to align the chromatograms, the method showed good reproducibility and standardization, considering the variation of the relative standard deviation (RSD) of the absolute retention times (ART) and the RRTs, which ranged from 0.68% to 0.97% and from 0.39% to 0.72%, respectively. The modifications of the HPLC protocol increased the discriminatory power by improving both the separation capability and the specificity of the method for mycolic acids. The chromatographic fingerprints generated by the adapted-HPLC were successfully used for identification of 24 Mycobacterium species from animal and human clinical samples including 21 members of the Mycobacterium tuberculosis complex and 3 members of the Mycobacterium chelonae-abscessus group.
Keywords: Mycolic acid separation, Mycobacterium spp. identification, M. tuberculosis complex, Mycobacterium massiliense, High performance liquid chromatography
Cite this paper: Leone Vinícius Furlanetto, Carlos Adam Conte-Júnior, Eduardo Eustáquio de Souza Figueiredo, Rafael Silva Duarte, Walter Lilenbaum, Joab Trajano Silva, Vânia Margaret Flosi Paschoalin, HPLC Protocol for Identification of Mycobacterium spp. from Clinical Samples of Human and Veterinary, Journal of Microbiology Research, Vol. 4 No. 6, 2014, pp. 193-200. doi: 10.5923/j.microbiology.20140406.02.
Article Outline
1. Introduction
- Since 1896, when Lehmann and Neumann described the bacterium responsible for causing tuberculosis and leprosy, about 150 species of Mycobacterium have been described. Except for M. leprae, which does not grow in vitro, those species were classified in two distinct groups: species that belong to the M. tuberculosis complex, and non-tuberculous mycobacteria (NTM) (Brasil, 2008; Euzéby, 2011).Mycobacterium tuberculosis infects over one-third of the human population worldwide, causing nine million new cases and two million deaths annually (WHO, 2014). While members of the M. tuberculosis complex cause more disease worldwide than any other bacteria (Dye, 2006), NTM are widespread in nature and, with some significant exceptions, are free-living saprophytes and opportunistic pathogens. Although considered to be non-pathogenic, NTM can pose a threat to humans, mainly in patients with underlying conditions such as AIDS or cancer, and there is an increasing awareness of their public-health importance, especially as nosocomial pathogens (Griffit et al., 2007).Differentiation of mycobacteria to the species level is currently performed by the time-consuming evaluation of phenotypic and biochemical characteristics (Furlanetto et al., 2012a). Different species can display distinct antibiotic resistances and require different prescriptions for treatment. For this reason it is important to identify Mycobacterium species in a rapidly and accurately way (Du et al., 2008; Furlanetto et al, 2012b).Complex high-molecular-weight β-hydroxyl fatty acids with a 22- or 24-carbon alkyl chain at the α-position are structural characteristics of mycolic acids, a type of fatty acid found in the Mycobacterium spp. cell wall. Several methods of fatty-acid analysis have indicated that mycolic acids are species- or group-specific (Butler et al., 1991). High-performance liquid chromatography (HPLC) analysis of mycolic acids has emerged as a reliable method for the detection of mycobacteria, because of the rapid and reproducible nature of the method and because the mycolic-acid elution spectrum observed for each mycobacterial species has generally been found to be unique, except for two species (M. bovis and M. tuberculosis) that share the same spectrum pattern (Hagen and Thompson, 1995). The HPLC method has been considered a standard test for chemotaxonomic classification and rapid identification of Mycobacterium species by the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov) since 1990, and has been reported to be more than 96% accurate compared with DNA probe tests (Butler and Guthertz, 2001). However, although it is well described and standardised (CDC, 1996), the HPLC methodology can be affected by several factors and must be optimised in accordance with local laboratory capabilities in order to assure accurate diagnosis. A customised database, using locally adapted protocols, must be developed in order to obtain chromatogram spectra from reference strains in the new analytical conditions, accrediting the local methodology and allowing accurate analysis of clinical samples.The purpose of this study was to adapt the CDC protocol to the apparatus available in our laboratory, and to optimise the HPLC methodology for identification of mycobacteria of human and veterinary interest. The clinical isolates were identified by comparing their chromatogram fingerprints with a database of the mycolic-acid chromatograms from 35 reference mycobacteria strains, and also comparing these spectra with other databases reported in the current literature.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
- Reference strains used were: M. agri (ATCC 27406), M. abscessus (ATCC 19977), M. aichiense (ATCC 27280), M. asiaticum (ATCC 25276), M. avium (ATCC 25291), M. bovis (ATCC 19210), M. bovis BCG Moreau (INCQS 00062), M. chelonae (ATCC 35752), M. chitae (ATCC 19627), M. chubuense (ATCC 27278), M. diernhoferi (ATCC 19340), M. flavescens (ATCC 14474), M. fortuitum (ATCC 6841), M. gadium (ATCC 27726), M. gordonae, chromotype I (ATCC 14470), M. intracellulare (ATCC 13950), M. kansasii (ATCC 12478), M. massiliense (CRM 0019) (Duarte et al., 2009), M. mucogenicum (ATCC 49650), M. neoaurum (ATCC 25795), M. nonchromogenicum (ATCC 19530), M. obuense (ATCC 27023), M. parafortuitum (ATCC 19686), M. peregrinum (ATCC 14467), M. phlei (ATCC 11758), M. porcinum (ATCC 33776), M. rhodesiae (ATCC 27024), M. scrofulaceum (ATCC 19981), M. simiae (ATCC 25275), M. smegmatis (ATCC 19420), M. terrae (ATCC 15755), M. triviale (ATCC 23292), M. tuberculosis (ATCC 25177 [(H37Ra)] and 27294 [(H37Rv]), M. vaccae (ATCC15483) and M. xenopi (ATCC 10042).Mycobacteria strains were cultured in Lowenstein-Jensen, except for M. bovis that was grown in Stonebrink media, at 35°C, following the recommendations of the Brazilian National Manual for the Laboratory Surveillance of Tuberculosis and other Mycobacteria (Brasil, 2008).
2.2. Mycolic Acid Sample Preparation
- Saponification of mycobacteria, extraction of mycolic acid and its derivatization to p-bromophenacyl were performed following the standard protocol for HPLC identification of mycobacteria (CDC, 1996). Briefly, 1–2 loops of each culture were transferred from the slant to a glass tube (13 by 100 mm) and 2 mL of methanolic saponification reagent (25% potassium hydroxide in 50% methanol) was added. The tube was capped tightly, homogenised and autoclaved for 1 h at 121°C and 15 psi. Free mycolic acids were extracted and derivatised to UV-absorbing bromophenacyl esters by acidifying with 1.5 ml of a 50% solution of concentrated HCl and H20 (v/v) and extracted from the mixture with 2 ml chloroform. The chloroform layer was dried under air at 80-100°C, and 2 mg of potassium bicarbonate was added. This preparation was resuspended in 1.0 ml chloroform, and 50 µl p-bromophenacyl-8TM reagent (Pierce Chemical Co., Rockford, IL, USA) was added. Derivatization was completed in a water bath at 80-100°C for 20 min. Tubes were cooled, the mixture was acidified with 1 mL of the acidification solution (concentrated HCl and H2O; 1:1, v/v), and 1 mL methanol was added. After the solution was thoroughly mixed, the bottom chloroform layer was transferred to a 1.8 mL glass tube and evaporated to dryness. Samples were resuspended in 50 µl methylene chloride before analysis.
2.3. HPLC Conditions
- Mycolic acids were analysed using a Shimadzu Prominence System modular HPLC apparatus (Shimadzu Co. Ltd., Kyoto, Japan) equipped with Shimadzu model LC-20AT pumps for stabilization of the gradient elution chromatography, a Shimadzu diode array detector model SPD-M20A set at 260 nm, and the software LC-Solution connected to a Shimadzu processor model CBM-20A. Samples were injected manually using an injector with a 20 μl sample loop. A Kromasil C-18 reverse-phase analytical cartridge column (4.6 mm by 10.0 cm packed with 3.5 μm silica was equilibrated in 98% phase A (95% methanol and 5% methylene chloride) and 2% phase B (5% methanol and 95% methylene chloride) with a flow rate of 2 mL min-1.During the first minute following the injection, the solvent concentrations were changed to 80% phase A-20% phase B, and then changed linearly at the 2nd min to 35% phase A-65% phase B. During the next 17.5 min the column was re-equilibrated in 98% phase A-2% phase B. The total run time was 20 min.
2.4. Chromatogram Spectra Analysis
- A training set of the 35 reference mycobacteria strains, chosen among the species most commonly isolated from clinical samples, were analysed by HPLC in our laboratory. The chromatographic pattern for each strain was examined for differences in the heights for pairs of peaks. HPLC patterns were grouped according to species, and the values calculated for each ratio were combined, sorted in numerical order, and examined for their ability to discriminate species, using the range of the relative standard deviation (RSD) of the absolute retention times (ART) and the relative retention times (RRT).
3. Results and Discussion
3.1. Adaptation of the HPLC Elution Program
- The elution program was adapted from the CDC (1996) protocol, mainly because the C-18 chromatography column used (10.0 cm) is 33% taller than those used in the old protocol (7.5 cm), resulting in a different retention time, even under the original elution program. The run time was increased to 20 min, which affected mainly the second step when the ratio of methylene chloride was increased and the long-chain mycolic acids, derivatised to p-bromophenacyl ester, were eluted at 17.5min, and the flow rate was decreased to 2.0mL min-1. Also, 5% methylene chloride was added to the methanol phase (A) and 5% methanol was added to the methylene chloride phase (B) in order to reduce bubbles and the drift of the baseline, as described by Du et al. (2008).The modifications that extended the run time, at the same time improved the separation capabilities and consequently increased the specificity of the method. Several peaks or clusters of peaks of the chromatograms from different strains showed better resolution than those previously described. As mentioned above, there is a wide range of structures and also concentrations of types or classes of mycolic acids (α, methoxy, keto, epoxy mycolates, etc.) among mycobacterial species. The HPLC methodology is unable to separate all the homologous series of mycolic acids, and for this reason the chemical composition of the chromatogram components could not be precisely identified. Although the individual mycolate cannot be identified, this is not necessary for identification of mycobacteria, since a species-specific chromatographic pattern is generated (Butler et al., 1991; Butler et al., 1992). Du et al. (2008) used a column with different dimensions from that specified (15.0 cm × 4.6 mm, 5 μm) and an also a different elution program (run time 30 min and 1.5 mL min-1 flow rate) from the CDC specifications (1996). However, they obtained chromatograms quite similar to those from the CDC protocol, although the discrimination specificity was less than that found in the present study.
3.2. Chromatogram Profile Database from Mycobacterium spp.
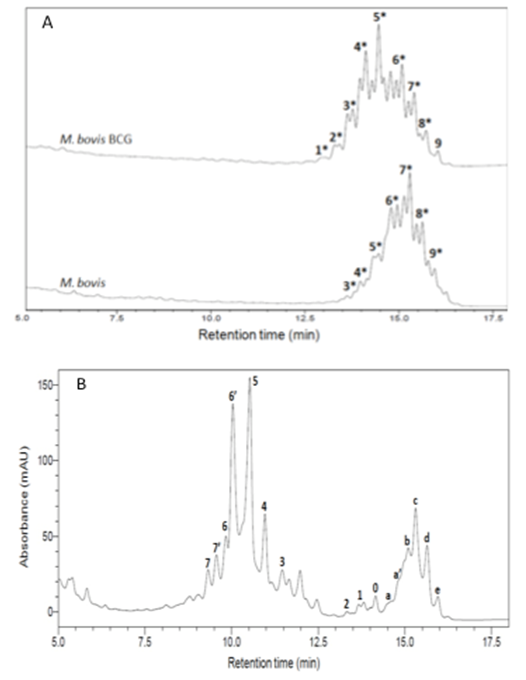
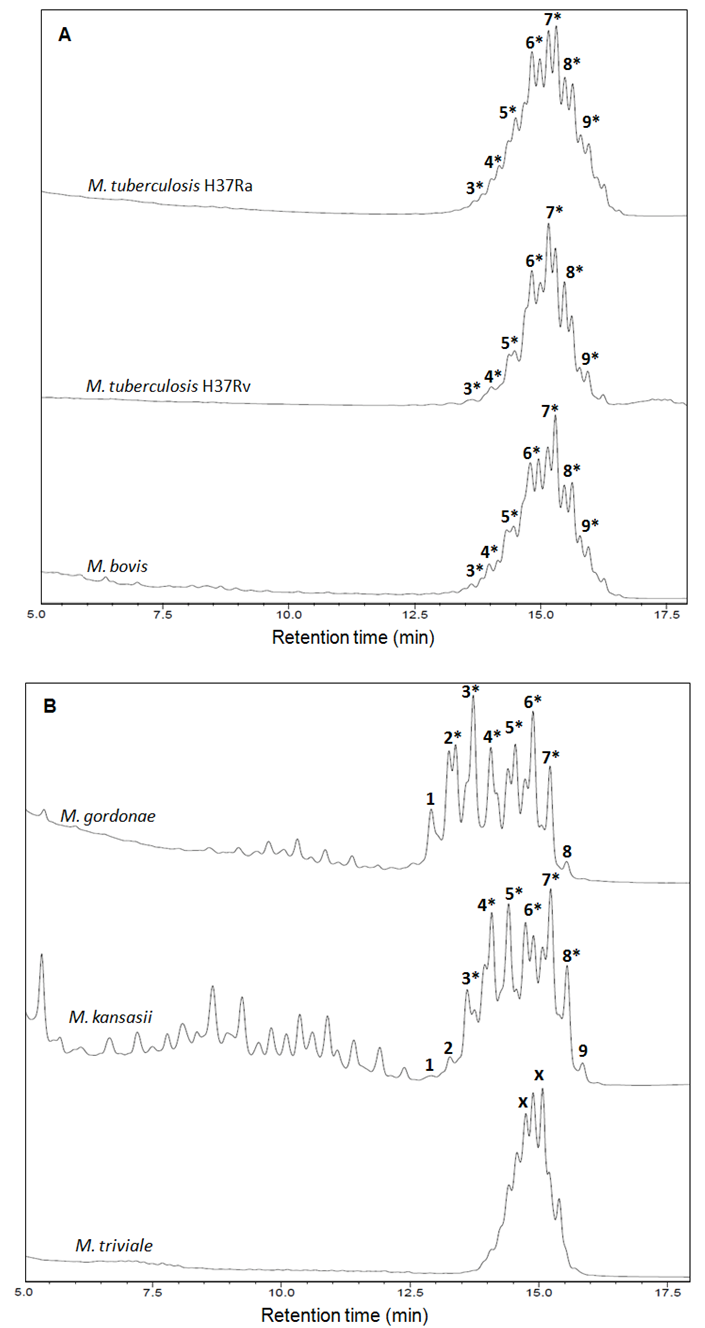
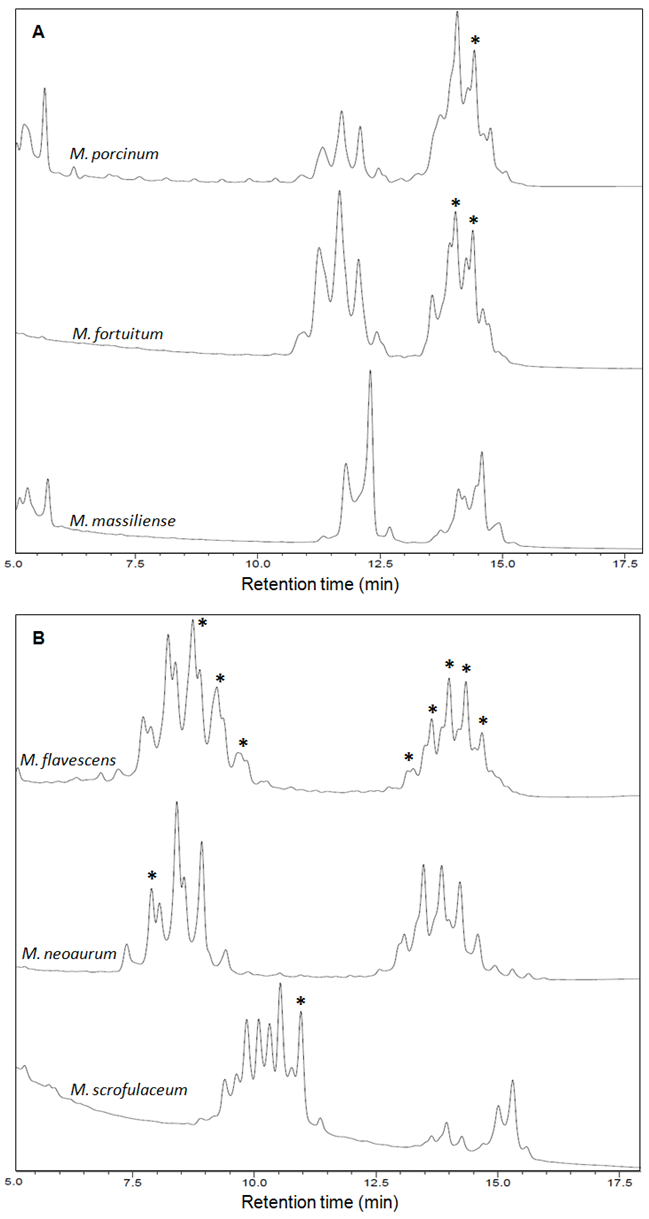
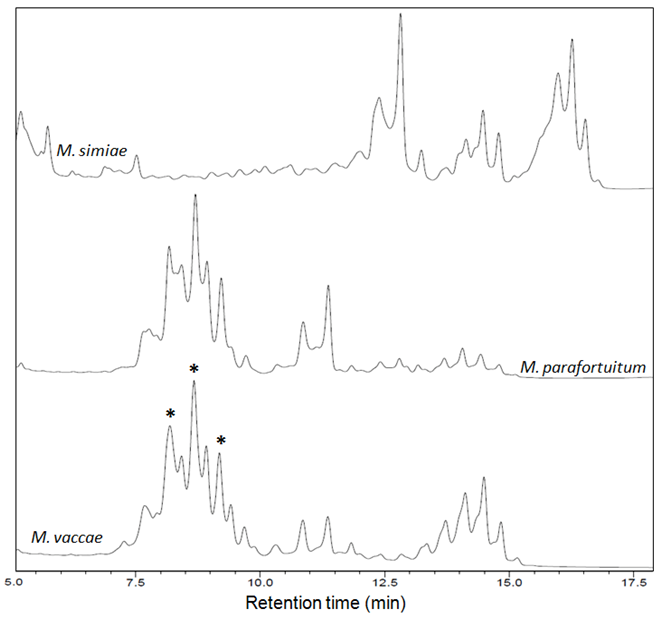
- The fingerprint library was constructed on the basis of HPLC chromatograms of the mycolic-acid derivatives from the 35 strains of Mycobacterium species that are most commonly found in clinical samples in our laboratory. The chromatogram fingerprints were grouped into three general patterns (single-, double- and triple-peak clusters) and divided in subgroups according to the chromatogram characteristics of mycolic-acid derivatives previously described by Butler and Guthertz (2001) (Figs. 1 - 4). RRTs were adjusted by comparison with external mycobacterial mycolic acid peaks (CDC, 1999). The M. intracellulare mycolic acid fingerprint (Fig. 1B) was used as a reference standard to help differentiate Mycabacterium spp., after the construction of the database.
3.2.1. Single-peak Cluster Patterns
- Members of the M. tuberculosis complex such as M. bovis and M. tuberculosis, and others species such as M. asiaticum, M. gordonae chromotype I and M. kansasii (Fig. 2B) showed chromatogram patterns with a single, late-emerging peak cluster. As mentioned above, the BCG-attenuated strains of M. bovis can be differentiated from the complex by the adapted-HPLC method (Figueiredo et al., 2012b).
3.2.2. Double-Peak Cluster Patterns
- M. chitae, M. porcinum (Fig. 3A) and M. agri are representatives of this group that displays late-emerging and close-together clusters of peaks. M. fortuitum (Fig. 3A), M. peregrinum and M. smegmatis are members of the M. fortuitum complex and displayed very similar chromatogram patterns. Therefore, the HPLC results obtained for these species provided insufficient information to distinguish between them.The M. chelonae-M. abscessus taxonomic group has undergone several revisions following the identification of newly recognised species such as M. massiliense (Fig. 3A), which was proposed in 2004, based mainly on genotypic analysis. As expected for closely related species (Duarte et al., 2009; Leao et al., 2009), all the members of this group showed a single chromatogram pattern.Some mycobacteria showed mycolic acid chromatogram patterns with widely separated double-peak clusters in the early-emerging cluster prior to 10.0 min. M. aichiense, M. diernhoferi, M. flavescens (Fig. 3B), M. gadium, M. mucogenicum, M. neoaurum (Fig. 3B), M. phlei and M. rhodesiae should be included in this cluster group.Strains showing those chromatogram patterns are hard to differentiate. M. avium and M. intracellulare (Fig. 1B), as well as M. nonchromogenicum and M. terrae showed very similar chromatogram patterns. M. scrofulaceum (Fig. 3B) and M. xenopi also fell within this group.
3.2.3. Triple-peak Cluster Patterns
- M. simiae could be included in this group, as shown in Fig. 4.
3.3. Reproducibility
- Nine different samples of M. intracellulare (ATCC 13950) were extracted and analysed independently, on three different days. Peaks 5, 4, c, d and e (Fig. 1B) showed RSDs of ARTs of 0.97, 0.94, 0.70, 0.69 and 0.68%, respectively. The RSDs of RRTs among these peaks ranged between 0.39 and 0.72%, indicating that the modified method has good reproducibility and standardization, even when a non-internal standard is used to align the chromatograms.
3.4. Identification of Mycobacterium spp. in Clinical Samples
- Identification of mycobacteria by the adapted-HPLC method was performed by comparing fingerprint patterns obtained from each clinical sample with those from the reference strains. The first criterion for identifying Mycobacterium spp. was to match the overall complexity and number of mycolic acid peak clusters: single, double and triple peaks. The second criterion was the range of time of elution between multiple peak groups, where the positions of peaks were determined as RRTs, adjusted by comparison to an external mycobacteria mycolic-acid peak. To increase reliability, the relationships of peak heights of major diagnostic peaks were determined and compared to those from reference strains.A total of 24 clinical isolated from animal and human samples were assayed by the adapted-HPLC in order to validate the methodology. Twenty-one of these mycobacterial were isolated from tissue, milk and nasal-swab samples collected from a dairy herd comprised of 270 adult crossbred Holstein and Gir cows, located in Macaé, Rio de Janeiro, Brazil. All isolates were identified as M. bovis (Fig. 5A-5B). The identity of these 21 mycobacteria was previously established by a multiplex PCR targeting for both RvD1Rv2031c and the IS6110 sequences (Figueiredo et al., 2009) and spoligotyping (Figueiredo et al., 2012a). Three isolates from clinical samples obtained from patients in Rio de Janeiro, Brazil, were identified as M. massiliense (Fig. 5C-5D) and confirmed after partial nucleotide sequencing of hsp65 or rpoB genes (Duarte et al., 2009).
ACKNOWLEDGMENTS
- The authors are grateful for financial support from FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) process number E-26/103.003/2012. L.V. Furlanetto and C.A. Conte-Junior were supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), process number 311361/2013-7.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML