Haba M. Abdelazez1, Entsar A. A. Nassar2, Khalid A. El-Dougdoug3
1Soils, Water and Environ. Res. Inst., Agric. Res. Center (ARC), Giza, Egypt
2Botany Department, Faculty of Science, Helwan University, Cairo, Egypt
3Virology Laboratory, Agricultural Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
Correspondence to: Khalid A. El-Dougdoug, Virology Laboratory, Agricultural Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
In a greenhouse experiment, was adapted to monitor potential of faba bean seed bacterization with R. leguminosarum for induction of systemic resistance in faba bean against Broad bean mottle virus and Botrytis fabae. The results demonstrated that BbMV or/and B. fabae challenged plants emerged from R. legumonisaruminoculated seeds not only showed a pronounced reduction in percent disease severity but also reduction in virus concentration and B. fabaecount compared to the non-bacterized ones. The bioproduct efficiency to restrict the pathogenicity of BbMV and B. fabaehardly exceeded 49.85 and 77.50% with seed bacterilization respectively. In parallel with disease reductions, the faba bean plant survival was the best (average of 71.0%) when Rhizo-N was seed bacterized system. Appreciable the significant increase in both salicylic acid level, chlorophyll content, protein and peroxidase activity were recorded in leaves of all PGPR (plant growth promoting rhizobacteria) inoculated plants compared to non-bacterized ones. At harvest, the tallest of nodulated plants (45.3 cm) were those received the bioagent Rhizo-N infected faba bean host 38.5 and 35.7 cm of BYMV and B.fabae. As well as 25,15 and 14 nodules -like structures per plant on their root system, respectively. On the other hand, BbMV and B.fabae induced systemic acquired resistant in faba bean plants against pathogens. Whereas mixed infection due to reduction of BbMV (49.85%) or B.fabae (49.80%) infection. It was increased by seed bacterization with 64.30% for BbMV and B.fabae. The nodulatedfaba bean plants; BYMV and B.fabae infected ones were varied in number and density of expressed protein bands. The polymorphic (specific bands) with 52.1% polymorphism, monomorphic (commonly bands) was percentage 34.2%. and the unique band (genetic markers) was percentage 13.8% related to 1 in Rhizobium(R), BYMV(V); 2 in B.fabae(B), R+V; 3 in R+B,V+B and 4 in R+V+B with MW 70 to 14 KDa.
Keywords:
BbMV, B. fabae, Acquired resistance, Salicylic acid, Enzyme activities, Faba bean
Cite this paper: Haba M. Abdelazez, Entsar A. A. Nassar, Khalid A. El-Dougdoug, Impact of Faba Bean Seed Bacterization with Rhizobium legumonisarum for Enhanced Plant Growth and Biocontrol Diseases, Journal of Microbiology Research, Vol. 4 No. 6, 2014, pp. 187-192. doi: 10.5923/j.microbiology.20140406.01.
1. Introduction
Faba been was on important legume food crop in ancient civilization and is still a major crop in many countries. In Egypt, the fab bean cultivated area declined up to 34% in 1993 due to an epidemic viral disease disaster in Central Egypt. Many viral and fungal diseases can affect faba bean plants and this considered a serious problem worldwide. Plant height leaf number, size, nitrogen fixed were found to reduce by viral and fungal infection [1].Viruses basically differ from other crop pathogens and pests because they cannot be eradicated chemically. Management of plant viral and fungal diseases can be accomplished through the induction of plant's natural defenses.Chocolate spot caused by B. fabae, is the most serious disease of beans and is capable of devastating an unprotected crop [2].The biological agents consisted of plant pathogenic bacteria, fungi, or viruses induced systemic acquired resistance (SAR) [3]. An alternative method to induce plant defense is through the use of non-pathogenic rhizobacteria which have the ability to induce a state of systemic acquired resistant, implants that, provides protection against a broad spectrum of phytopathogenic microorganisms. This approach has been referred to be induced systemic resistance [4]. Several Bacillus species elicit significant reductions in the incidence or severity of various diseases (leaf spotting fungal, bacterial pathogens, systemic viruses and root-knot nematodoes) on a diversity of hosts [5]. Pseudomonas determinants that are claimed to produce ISRs include siderophores, the O-antigen of heteropolysaccharides and salicylic acid. The latter compound has even been indicated to cause an ISR which present in nanogram amounts [6].In this study, we therefore investigated the response of faba bean plants subjected to inoculation with R. legumonisarum to BbMV and B. fabae infection.
2. Material and Methods
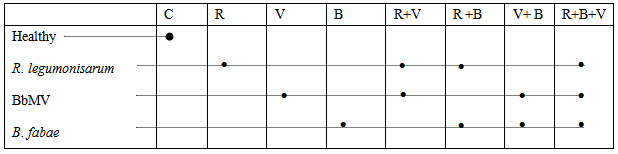
Bacteria inoculum: R. Legumonisarum isolate (obtained from bioferillizar Unit, Fac. Agric Ain Shams Univ.) was grown for 3 days in yeast mainnitol broth at 28℃ on rotary Shaker at 150 rpm. Late log phase cells were harvested by centrifugation (6.000 rpm at 4℃ for 10 min and washed twice with sterile NaCl solution (0.85%). Growth was monitored by absorbance measurements of culture sample at 550 nm. Cell densities were related to viable cell numbers, measured as colony forming unites per mL (CFU ml-1) by standard plate counts and the number of broth bacteria was adjusted to 108 CFU ml-1.Virus inoculum: BbMV isolate was obtained from Virology Lab. Microbiol. Dept. Fac. Agric. Ain Shams Univ. It was maintained on Faba bean and checked on Chenopodium amaranticolor. It was reisolated from single local lesion and inoculated on healthy faba bean seedlings CV. Giza. 401. The virus inoculum was prepared by grinding infected leaves in 100 ml phosphate buffer (pH 7.5) and centrifuged at 5000 rpm for 10 min. The supernatant containing virus was diluted at 10-1 in phosphate buffer and used as virus inoculum.Fungus inoculum: B. fabae was obtained from plant Path. Dept. Fac. Agric. Ain Shams Univ. It was propagated for 7 days at 25℃ on a rotary shaker at 150 rpm. The culture was harvested and filtrated to exclude mycelium growth. The conidia were washed twice with sterile NaCl saline solution (0.85%). The conidia were counted by a haemocytometer slide. The conidial suspension was adjusted at about mean density of 1010conidiospores ml-1 according to Chamber and Scott [7].Seed bacterization: Faba bean seeds (Viciafaba L. CV Giza 1) were surface sterilized by placing seeds in 2.5% sodium hypochlorite for 5 min. followed by rinsing in 1:29 mixture of hydrogen peroxide: distilled water for 30 min. and dried. The sterilized seeds were steeped in R. leguminosarum suspension mixed with 10% gum arabic for 1 hr and dried at room temperature. Seeds steeped in gum Arabic only as a control.Faba bean-biopreparate-parthogen panorama:Pot experiment: It was carried out to evaluate PGPR (R. leguminosarum) for inducing SAR in plants against BbMV and B. fabae. Bacterized and non-bacterized healthy. surface sterilized seeds were sown in earthen pots (20 cm diameter) containing clay soil. The pots were maintained in greenhouse under natural lighting; day/night temperature of approx. 26/15℃ and 60 mean relative humidity. The three leaves old faba bean seedlings in V; R+V, V+ B and R+B+V treatments (Table 1), in addition to the challenged control (CHC) were challenge-inoculated with BbMV. On the other hand B, R+B+V and RBV treatments were infected with B. fabae. At the beginning the experimental Layout was randomized complete block design was used with 8 treatments (Table 1) and each consisted of 5 replicate pots and 4 plants per pot. Treatments included plant healthy (non-inoculated with bacteria and virus) (C); R. leguminosarum (R), B. fabae (B); BbMV (V); (B + V), (R + V); (R + B) and (R + B + V). This experiment was repeated three times.Table (1). Layout of the experimental design
 |
| |
|
Disease incidence: After 45 days from planting, the number of plants exhibited B. fabae and BbMV symptoms was recorded. Percent of disease incidence (PDI) was estimated according to the equation: PDI = (Number of symptomatic plants / total number of plants) X 100. The efficiency of biopreparate to control pathogenic fungi and virus were determined using the where: A = disease percentage of bioproduct an treated plants, B = disease percentage of treated ones. Besides, the percentages of seedling survival were determined for those left to grow up to 40 days, nodule formation on faba bean root system was monitored as well. DAS-ELISA: BbMV was detected by double antibody sandwich enzyme linked immunosorbent assay (DAS-ELISA) according to Clark and Adam [8]. Five plants from each treatment and six leaves from each plant were used as replicates to carry out ELISA determination. Absorbance values were recorded using a Dynatech MR 700 plate reader at 405 nm.Salicylic acid (SA): Two leaves from each treatment were frozen in liquid nitrogen and pulverized with mortar and pestle. For determination of SA. 200 mg of the standard ortho-anisic acid was added per gram of fresh leaves. Subsequently, extraction and quantification of free SA expressed as μg-1 fresh leaves were carried out as described by Meuwly and Métraux [9].Peroxidase activity (POA): One gram fresh weight of leaf tissue was ground in a mortar containing liquid nitrogen. The resulting powder was macerated for 30s in 10 ml of 0.1 M tris buffer, pH 7.5 and then centrifuged at 20.000 g for 25 min at 4℃. The supernatants were kept in an ice bath and used for determination of (POA) activity by a direct spectrophotometric method described by Hammerschmidt et al. [10]. POA activity as expressed as absorbance changes min-1 g-1 fresh leaf tissue at 470nm. SDS-PAGE: Leave proteins finger print was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli [11] as modified by Studier [12]. The revealed banding profile of different treatments were qualitatively and quantitatively determinate in order to detect the acquired resistance related to PGPR against BYMV and B.fabae infection.
3. Results
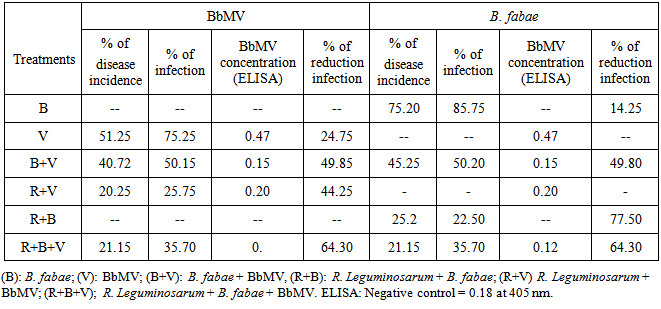
In the present study, the response of faba bean plants emerged from seeds bacterized with R. leguminosarum to challenge with BbMV and B. fabae is quantified in table 2and 3. Challenged plants emerged from R. leguminosarum inoculated seeds (R+B), (R+V) and (R+B+V) treatments showed pronounced and significant reduction in percent disease incidence (20.25); (21.15); (25.21) and (15.75%) in (R+V), (R+B+V); (R+B) and (R+B+V) treatments respectively. Compared with 51.25 and 65.20% for the challenged control plants with BbMV and B. fabae respectively. Also inoculation with R. leguminosarum (R) significantly reduced BbMV concentration in the challenged plants with BbMV (R+V), (V+B) and (R+V+B) whereas ELISA values of 0.20,0.15 and 0. While the challenged control (V) showed on ELISA value of 0.47 (table 2). On the other hand, seed bacterization due to reduction of B. fabae incidence and infection were 25.2 and 77.5 compared with 75.2and 85.75% for the challenged control plants with B. fabae (Table 2).Table (2). Quantification of R. leguminosarum mediated induced systemic resistance to BbMV and B. fabae infection
 |
| |
|
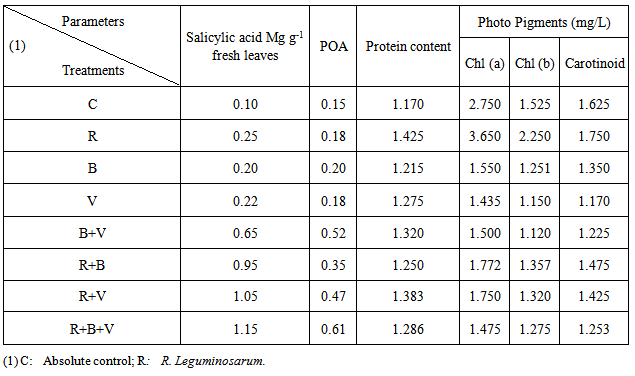
In challenged treatments, significantly higher salicytic acid (SA) level of 1.15 μg g-1 fresh leaves was recorded in leaves of (R+V+B) treatment followed by R+V, 1.05 and R+B, 0.95 μg g-1 fresh leaves, compared with 0.22; 0.20 and 0.10 for V, B and C treatments respectively (Table 3). The salicylic acid level was further increased when seed were inoculated with R. leguminosarum along with BbMV and B. fabae and showed 0.25; 0.22; and 0.20 μg g-1 fresh leaves respectively (Table 3).Table (3). Quantification of R. leguminosarum mediated induced systemic resistance to B. fabae and BbMV in 45 days old faba bean plants
 |
| |
|
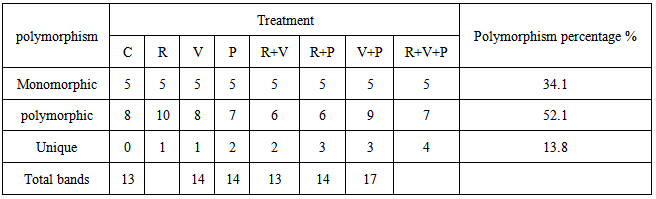
Regarding the peroxidase activity (POA), an appreciable and significant increase was observed implants if all R. leguminosarum – inoculated treatments compared to the other treatments. R. leguminosarum (R) moculate of plants showed a very low POA, equal to that in plants of challenged (V) and (B) treatments. It was also observed that inoculation of Faba bean seeds R. leguminosarum increased both SA and POA in emerged plants independently whether the plants were challenged with BYMV or/and B. Fabae, but the increase in challenged plants was more pronounced (Table 3). The data present in table (3) show that inoculation with R. leguminosarum improved plant performance in terms of photo pigments (Chla, Chlb and carotenoids) content as compared to unchallenged control plants. However the inoculated plants showed the best performance. Challenging with BYMV decreased drastically Chl a, Chlb and carotenoids of faba bean plants emerged from non-inoculated seeds. Moreover, R. leguminosarum inoculated challenged plants (R+V),( R+B) and (R+B+V) treatments showed pronounced improvement in photo pigments 1.750,1.772,1.450 (Chl. a);1.320,1.357,1.275 (Chlb) and1.425,1.475,1.252 (carotenoids), respectively (table 3).The result recorded in table (3) has higher value of protein whereas R. leguminosarum inoculated plants (R) has higher value of protein contant (1.425) followed by 1.175; 1.215; 1.275; 1.320; 1.250, 1.383 and 1286 for (C), (B), (V); (B+V); (R+B); (R+V) and (R+B+V) treatment, respectively.SDS-PAGE profile of protein pattern (Fig. 1) extracted from faba bean plants inoculated with R. leguminosarum challenged with B. faba and BYMV and non-challenge plants as control illustrated in Fig. (1). It was revealed bonds with variable molecular weight, number, and density among (C), (R), (B), (V), (B+V), (R+B); (R+V), (R+B+V) treatments. The variability analysis among treatments showed some polypeptides (polymorphic) absent or/and present in some treatments (Fig. 1) with 52.1% percent. Polymorphic polypeptides appearance may attribute to the influence of each-treatment on host plant. Some polypeptides appeared in all treatments (common polypeptides) monomorphic with 34.2% percent. These polypeptides related to faba bean plants. Other polypeptides are genetic markers(unique band) with 13.8% related to 1 band in R.V; 2 bands in B,R+V; 3 bands in R+B,V+B and 4 bands in R+V+B treatments (table 4). These band may be related to rhizoibia, viral, or fungal protein.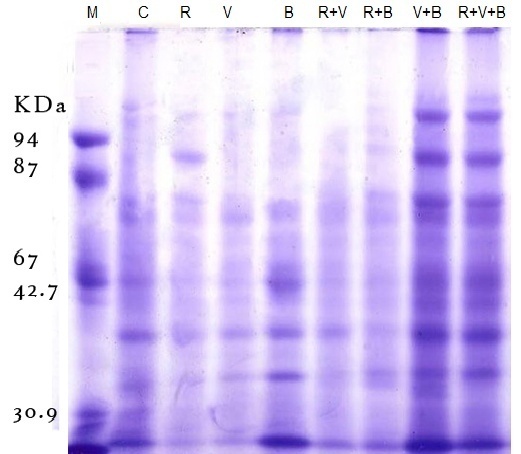 | Figure (1). SDS-PAGE,12 % of total protein analysis showing polypeptide bands of inoculated faba bean plants with R leguminnd infected with BYMV and B. fabae under greenhouse condition |
Table (4). Polymorphism and genetic markers in leaves of nodulated faba bean plants infected with BYMV and B. fabae
 |
| |
|
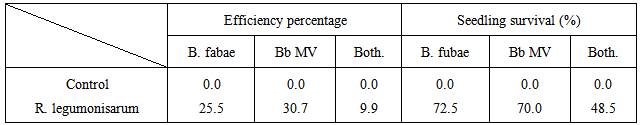
The seed bacterilization tested considerably varied in respect to their efficiency in Fungal and viral pathogenicity restriction (table 5). In general R. legumonisarum (seed bacterilization preparation) was the most efficient of symptoms severity reduction 30.7 (BYMV), 25.5 (B. fabae) and 9.90 (V+B). In addition, increased seedling survival percentage 70.0,72.5 and 48.5%, respectively.Table (5). Efficiency percentage of biopreparate to alleviate the symptoms severity and faba bean survival percentage due to bioagents and pathogens treatments
 |
| |
|
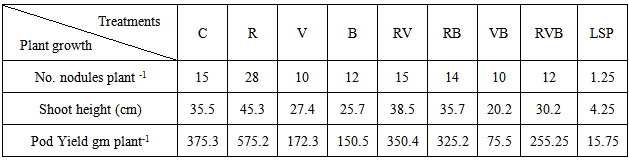
At harvest the height faba bean plants (45.3, 38.5, 35.7 and 30.2 cm) were those received Rhizo-N, and BbMV, B.fabae, BbMV+B.fabae respectively compared with non-receviod Rhizo-N, BbMV, B. fabae, and BbMV+ B.fabae 35.5, 27.4, 25.7 and 20.2 cm, respectively (Table 5). Faba bean root out growth nodules ranged from 12 to 28 nodules per plant root were received Rhizo-N. Untreated legume hosted the lowest number of ranged from 10 to 15 nodules per plant (Table 5). The legume pod yield significantly stimulated due to application of seed-bacterilization (Table 5). Plants received no bioagent produced 275.3 gm plant-1 pod yield increases in treated plants reached 575.2, 350.4; 325.2 and 255.25 gm, R,(R +V), (R+B ) and (R+V+B) treatments, respectively. Compared to 172.3; 152.5, 75.5 gm plant-1 pod yield of V; B and (V + B) treatments respectively. Table (6). Faba bean plant growth treated with Rhizo-N infected with B. fabae and /or BbMV under greenhouse conditions
 |
| |
|
4. Discussion
The response of faba bean plants (Viciafaba CV. Giza 401) emerged from seeds bacterized with R. leguminosarum strain in the rhizosphere and the foliar pathogens BYMV and B. fabae in the phyllosphere satisfy the condition of non-specific protection proposed by Steiner and Schonbeck [13] as a criterion of induced systemic resistance (ISR). The onset of ISR was assayed as a significant reduction in both PDI (20.25; 25.21 and 15.75) and BYMV concentration (0.47) relative to challenged control (51.25; 75.25; 40.72 and 45.25) and (0. and 0.20 ELISA value), respectively.Rhizobium species are widely used in agriculture of legume crops improvement because of their ability to fix atmospheric nitrogen in symbiosis with legumes. The results show that inoculation of faba bean seeds with Rhizobium induced SR in faba bean plants against BYMV and B. fabae is very interesting. The bacterial lipopolysaccharides (LPS) appeared to be the trait responsible for systemic resistance induction by Rhizobium rhizobacteria to plant pathogenic on the other hand, surface carbohydrates of Rhizobium rhizobacteria cells consist mainly of exopolysaccharides (EPS). The polysaccharides play an important role during the recognition process in the symbiotic interaction between Rhizobium and Legumes [14-16]. However, further studies are needed to support this finding that inoculation with rhizobia could induce SR in legumes against pathogen infection. Choudhary and Johri [5], reported that several strain and species of Bacillus elicit significant reductions in the incidence or severity of various diseases on a diversity of hosts. Protection resulting from induced systemic resistance (ISR) elicited by Bacillus spp. has been reported against leaf spotting fungal and bacterial pathogens, systemic viruses, a crown-rotting fungal pathogen, root-knot nematodes, and a stem-blight fungal pathogen.The PGPR medicated SR is often associated with the onset of defense mechanism including the increased expression of defense mechanism including the increased expression of defense enzymes, such peroxidase [16, 17]. In the present study increased peroxidase activity was observed in R. leguminosarum inoculated plants.Appreciable and significant increase in both SA level and peroxidase activity was observed in leaves of all PGPR inoculated plants compared to other treatments. Since the PGPR inoculants (Pseudomonas and Rhizobium) and the pathogen (BYMV) remained spatially separated, it can be concluded that the tested Pseudomonas or Rhizobium strains induced systemic resistance in faba bean against BYMV [16].In conclusion under out experimental conditions the rhizobacterium R. leguminosarum seems to be a promising inducers for SR in faba bean to challenge BYMV or/and B. fabae infection under greenhouse conditions. This result could be very important partically since it may offer a simple environmentally safe and economically accepted mean to protect faba bean plants from BYMV or B. fabae infection. However, additional studies are needed to confirm these results under field conditions.Leave proteins finger print was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli [11] as modified by Studier [12]. The revealed banding profile of different treatments were qualitatively and quantitatively determinate in order to detect the acquired resistance related to PGPR against BYMV and B. fabae infection.Protein expression, in general our results revealed that BYMV and B. fabae infected nodulated faba bean plants lead to increase in the protein content of leaves. It can be interpreted by the fact that the biotic inducers increased performance of RNA machinery. The increase in protein was appeared by SDS-PAGE technique as increase in polypeptide bands.SDS-PAGE is a widely used technique for the separation and MW weight estimation of individual polypeptids [18-20]. It used in detection of genetic expression of induced genes under Rhizobium inoculation, viral and fungal infection (a biotic inducers). Where it appeared distinct differentiation for faba bean plants under different a biotic inducers not only in healthy but also in infected ones. Specific expression of induced proteins is an important adaptive manifestation in maintaining the integrity, native configuration and topology of cellular membranes components to ensure their normal functioning under a biotic stress [18-20]. The increase in number of polypeptides suggests that BYMV and B. fabae effect on protein activities in faba bean plants. A negative relation was found between cell membrane stability and protein contents. Changes in the expressed protein occur due to biotic, yet it is probable that only some of these proteins are directly involved in infected plants tolerance. It is possible that in some cases the synthesis of a protein indicates sensitivity to a stress rather than being part of a tolerance mechanism. The increase of polypeptides in nodulated faba bean plants, irrespective of their infection tolerance.
References
| [1] | Mona, H. Badawi, Hydi, M. El-Henawy and Abd-Elghaffar, N.Y. (2013). Biomanagement of Fusarium Solani and Rhizactomiasdani causing root rot and damping off diseases in common bean (Phaseolus vulgaris) via innovative rhizobacterial formulations. Journal of Applied Sciences Research 9 (1): 321-329. |
| [2] | Khaled, A.A.; Abd El-Moity, S.M.H. and Omar, S.A.M. (1995). Chemical control of some faba bean diseases with fungicides. Egypt. J. Agric. Res. 73(1) 45-56. |
| [3] | Ryals, J.; Uknes, S. and Ward, E. (1994). Systemic acquired resistance. Plant Physiol. 104, 1109-1112. |
| [4] | Van Loon, L.C.; Bakker, P.A.H.M. and Pieterse, C.M.J. (1998). Systemic induced resistance by rhizosphere bacteria. Annu. Rev. Phytobathol. 36, 453-483. |
| [5] | Choudhary D.K and Johri, B.N. (2008). Interactions of Bacillus spp. and plant with special reference to induced systemic resistance (ISR). Microbial Res; 10: 08-017. |
| [6] | De Mayer, G.; Capieal, K.; Audendert, K.; Buchala, A.; Metraux, J.P. and Hofte, M. (1999). Nanogram amounts of salicylic acid and produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 active the systemic acquired resistance pathway in bean. Mol. Plant. Microbic Interact. 12, 450-458. |
| [7] | Chamber, S.M. and Scott, E.S. (1995). In vitro antagonism of Phytaphothera Cinnamomi and P. Citricolaby isolated of Trichoderma spp. and Gliocladiumvireus. Rhytopath. 143: 471-477. |
| [8] | Clark, M.F. and A.N. Adams., 1977. Characterization of the microplate method of enzyme-linked Immunosorbent assay for the detection of plant viruses. J. of Gen. Virology., 33: 475-483. |
| [9] | Meuwly P and Métraux JP (1993). ortho-Anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem 214: 500-505. |
| [10] | Hammerschmidt R., E.M. Nukles and J. Kuc., 1982. Association of enhanced peroxidase activity with induced resistance of cucumber to Colletotrichumlagenarium. Physiological Plant Pathology 20: 73-82. |
| [11] | Laemmli UK (1970). Cleavage of structural head of bacteriphage. Nature; 227: 680-685. |
| [12] | Studier FW (1973). Analysis of bacteriophage T, early RNAs and proteins of slab gel. Journal of molecular Biology; 79: 237-248. |
| [13] | Steiner, U. and Schonbeck, F. (1995). Induced disease resistance in monocots. In: R. Hammerschmidt. J. Kuc (eds.): Induced Resistance to Disease in Plants, pp. 86-110, Kluwer, Dordrecht, The Netherlands. |
| [14] | Denny, T.P. (1995). Involvement of bacterial polysaccharides in plant pathogenesis. Ann U. Rev. Phytopathol. 44, 173-197. |
| [15] | Leigh J.A. and Coplin, D.L. (1992). Exopohysaccharides in plant-bacterial interactions. Ann. Rev. Microbial. 46, 307-346. |
| [16] | Elbadry, M.; Taha, R.M.; El Dougoug, K.A.; and Gamal-Eldin, H. (2006). Induction of systemic resistance in faba bean (Viciafaba L.) to Bean yellow mosaic (BYMV) via seed bacterization with plant growth promoting rhizobacteria. Journal of Plant Diseases and Protection, 113(6): 247-251. |
| [17] | Bergstrom, G.C.; Johnson, M.C. and Kuc, J. (1982). Effects of local infection of cucumber by Colletotricham Legenadium, Pseudemonas Lachrymansor Tobacco necrosis virus on systemic resistance to cucumber mosaicvirus. Phytopathology 72, 922-926. |
| [18] | Mohamed, E.F. (2011). Changes in protein, amino acids composition and leaf cells of beet plants (Beta vulgaris L) due to Beet mosaic virus (BtMV) infection. Journal of American Science. 7:245-254. |
| [19] | Sofy AR, Attia MS, Sharaf AMA and El-Dougdoug KA (2014a). Bean yellow mosaic potyvirus potential on nodulation and N2-fixation of faba bean plants. N Y Sci J 2014;7(9):101-109. |
| [20] | Sofy AR, Attia MS, Sharaf AMA and El-Dougdoug KA (2014b). Potential impacts of seed bacterization or salix extract in faba bean for enhancing protection against bean yellow mosaic disease. Nat Sci 2014;12(10):67-82. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




