-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(5): 180-182
doi:10.5923/j.microbiology.20140405.02
Medicinal Efficacy of Methanol and Ethanol Crude Extracts of Mangifera indica Leaf
Iroha Ifeanyichukwu1, Ejikeugwu Chika2, Nwakaeze Emmanuel1, Oji Anthonia1, Afiukwa Ngozi1, Nwuzo Agabus1
1Department of Applied Microbiology, Ebonyi State University, Abakaliki, Nigeria
2Department of Pharmaceutical Microbiology and Biotechnology, Nnamdi Azikiwe University, Awka, Nigeria
Correspondence to: Ejikeugwu Chika, Department of Pharmaceutical Microbiology and Biotechnology, Nnamdi Azikiwe University, Awka, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was to determine the antibacterial activity of methanol and ethanol leaf extracts of Mangiferaindica against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Klebsiella pneumoniae. Antibacterial activity of the methanol and ethanol leave extracts of M. indica was determined by the agar well diffusion method at a concentration of 100 mg/ml while minimum inhibitory concentration (MIC) was determined by the agar dilution technique. The results obtained in this study showed that the ethanol and methanol crude extracts of M. indica leafhad antibacterial activities and inhibited the test pathogens substantially. However, methanol extract produced better zones of inhibition than the ethanol extracts. The zones of inhibition of both extracts ranged between 10-16 mm. Minimum inhibitory concentration (MIC) of the ethanol and methanol M. indica leaf was determined at 100 mg/ml and 50 mg/ml against all test isolates. In conclusion, this preliminary study has shown that M. indica possess potent antibacterial activity and this underscores the reason why the plant is used to meet a number of primary health care needs in most rural Nigerian communities.
Keywords: Plants, Herbal, Magnifera, Resistance, Nigeria
Cite this paper: Iroha Ifeanyichukwu, Ejikeugwu Chika, Nwakaeze Emmanuel, Oji Anthonia, Afiukwa Ngozi, Nwuzo Agabus, Medicinal Efficacy of Methanol and Ethanol Crude Extracts of Mangifera indica Leaf, Journal of Microbiology Research, Vol. 4 No. 5, 2014, pp. 180-182. doi: 10.5923/j.microbiology.20140405.02.
1. Introduction
- Mangifera indica is a large evergreen tropical tree in the family Anacardiaceae, and with a heavy dome-shaped crown that has horticultural and medicinal applications (Dweck, 2001). Its fruits contain protein, fats, carbohydrates, minerals, vitamins and amino acids; and saponins, glycosides, unsaturated sterols, and polyphenolsare some of the important active constituents that characterize the plant (Dweck, 2001). Indigenous plants including M. indica have been used since antiquity by humanity to treat a handful of infectious diseases (Stockwell, 2004). M. indica has many antimicrobial effectiveness that warrants its usage in the management of microbial related diseases and even non-microbial ailments in many parts of Africa (Aderibigbe et al., 2001; Aderibigbe et al., 1999; Amrita et al., 2009). Aside its medicinal applications, M. indica are widely used as a source of food and timber in the parts of the world where it grows (Bala, 2006). Generally, plants especially those with proven antibacterial activity (e.g. M. indica) produce a wide variety of secondary metabolites which can sum up to important sources of active constituents for the production of potent pharmaceuticals including drugs that can be used to assuage the effects of antimicrobial resistance in clinical medicine (Garrido et al., 2001; Khan et al., 2003). It is no longer news that the indiscriminate use of antibiotics has opened the window for resistant strains of microbes to emerge and spread in both the community and hospital settings. It has been well reported that medicinal plants are natural and potent sources of pesticides, microbicides, and antimicrobials that could be used for the effective control of pathogenic microorganisms (Duke, 2007; Garrido et al., 2004; Ojewole, 2005; Bidla et al., 2004). If this is the case, these medicinal plants including those of M. indica could be harnessed to develop novel antimicrobial agents for the treatment of microbial related diseases. The primary benefits of using plant-derived medicines are that they are relatively cheaper than synthetic alternatives, offering profound therapeutic benefits and more affordable treatments. Many of the plant materials used in traditional medicines are readily available in rural areas and this has made traditional system of medicine relatively cheaper than modern medicine in this part of the world. This necessitates the need for the continued screening of medicinal plants, not only to determine the scientific basis for their usage, but also to discover new active constituents for the development of novel drugs. This study investigated the antibacterial activity of leaf extracts of M. indica plants against clinically important bacterial pathogens.
2. Materials and Methods
- Collection and identification of plant materials: Fresh stem, bark and leaves of Mangifera indica were collected from Ntezi village in Ebonyi local government area of Ebonyi State, Nigeria in the month of May, 2013 and were identified by a taxonomist, Prof. S.C.C. Onyekwere of Applied Biology Department, Ebonyi State University, Abakaliki, Nigeria.Preparation of plant materials: The freshly collected leaves of Mangifera indica were washed under running tap water and dried in air for 5 days. The leaves were homogenized to fine powder using a grinding machine. The powder was stored in airtight container until use (Vaghasiya et al., 2000).Extraction and preparation of extracts: Twenty grams of the fine powder of the grinded M. indica leaves was immersed in 95% ethanol and methanol for the extraction of its active constituents according to a previously used methodology (Falodun et al., 2006; Vaghasiya et al., 2000). The crude extract was then filtered using muslin cloth and Whatman No.1 filter paper; and the filtrate was allowed to evaporate to dryness using rotary evaporator (Model 349/2, Corning limited) at 40℃. The dried substance was stored in airtight bottles until required. For the preparation of dilutions of crude extracts for antimicrobial screening, a reconstituted ethanolic extract was prepared by dissolving 20 mg in 1 ml of ethanol to obtain a concentration of 100 mg/ml. This procedure was also used in methanol extraction.Inoculum preparation and determination of antibacterial activity: Pure cultures of the test organisms including Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus were obtained from the Department of Microbiology of the Federal Teaching Hospital Abakaliki (FETHA), Ebonyi State, Nigeria. The antibacterial activity of the M. indica extracts was determined by agar well diffusion method on nutrient agar plates as was previously described (Vaghasiya et al., 2000; Ojewole, 2005). Briefly, wells were made on the agar plates using 6 mm cork borer and the test organisms (adjusted to 0.5 McFarland turbidity standards) were each inoculated on the medium and allowed for about 10 minutes. Equal concentrations of the extracts (50 mg/ml) were introduced into each of the wells. Ampicillin was used as the positive control drug. Plates were incubated overnight at 37℃ and observed for zones of inhibition.Minimum inhibitory concentration (MIC): Minimum inhibitory concentration (MIC) is the lowest concentration of the plant extract(s) that was able to inhibit the growth of the test organisms. MIC was determined by the agar dilution method at varying concentrations of 100, 50, 25, 12.5 and 6.25 mg/ml using previous methodology (Ojewole, 2005; Vaghasiya et al., 2000).
3. Results
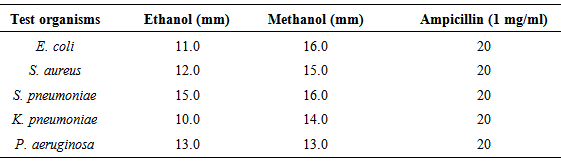
- Results of the antibacterial activity of the plant extracts on the test organisms are shown in Tables 1 and 2. The methanolic and ethanolic extracts of M. indica showed appreciable level of antibacterial activities against the test isolates compared to the control drug (ampicillin). The inhibition zones of the ethanol plant extract against the test organisms was 11 mm (E. coli), 12 mm (S. aureus), 15 mm (S. pneumoniae), 10 mm (K. pneumoniae) and 13 mm (P. aeruginosa). Compared to the standard test drug (ampicillin, 1 mg/ml), the ethanol extract of M. indica showed considerable antibacterial activity against the test bacteria. The methanol extract of M. indica showed varying rates of antibacterial activity against the test organisms at inhibition zones of 16 mm (E. coli), 15 mm (S. aureus), 16 mm (S. pneumoniae), 14 mm (K. pneumoniae) and 13 mm (P. aeruginosa). Methanol extract of M. indica showed better inhibition zones than the ethanol extracts of the plant (Table 1). The results of the minimum inhibitory concentration (MIC) of the ethanol and methanol extracts of M. indica indicate that the growth of the test organisms was inhibited at 100 mg/ml and 50 mg/ml (Table 2).
|
|
4. Discussion
- M. indica has been used in most rural communities for the treatment of a handful of infectious diseases due to its apparent medicinal properties that stands it out from other herbal plants. In this study, we evaluated as a preliminary examination the possible antibacterial activity of M. indica as a way to give credence to its wide usage in most rural communities in Abakaliki, Ebonyi State, Nigeria. Table 1 presents the results of the antibacterial activity of M. indica on clinically important bacterial pathogens including S. aureus, E. coli, P. aeruginosa, K. pneumoniae, and S. pneumoniae. It can be inferred from our results that both the ethanol and methanol leave extracts of M. indica had a broader spectrum of activity against the test isolates. However, better antibacterial activity was recorded with the methanolic extracts at different zones of inhibition. It could therefore be deduced from this study that methanol is a better extractor of the bioactive constituents of the M. indica leaves than the ethanol solvents used. Our result showed that ethanol extract of M. indica had activity on S. aureus (12 mm), S. pneumoniae (15mm), E. coli (11mm), K. pneumoniae (10mm) and P. aeruginosa (13mm). Methanolic extracts of M. indica also showed varying levels of activity on S. aureus (15 mm), S. pneumoniae (16mm), E. coli (16mm), K. pneumoniae (14mm) and P. aeruginosa (13mm). The notable antibacterial activities of M. indica on bacterial pathogens have been previously reported and these are also in line with the results obtained in this study (Aderibigbe et al., 2001; Aderibigbe et al., 1999; Amrita et al., 2009; Garrido et al., 2001; Khan et al., 2003). The MIC of the ethanol and methanol extracts of M. indica on the test isolates was determined at 100 mg/ml and 50 mg/ml. The antibacterial activity of M. indica leaf extracts which obviously tell their bioactivity on the test isolates is encouraging owing to the fact that these organisms are of hospital origin. The ability of the crude extracts of M. indica leafs to inhibit the growth of pathogenic bacteria (as obtainable in this study) is an indication that M. indica is a medicinal plant and could serve as a source of lead bioactive compounds for the development of potent antimicrobial agents. This further validates its use in traditional herbal medicine in most rural Nigerian communities to treat a variety of bacterial related infections including those associated with respiratory tract infections.
5. Conclusions
- This study have shown that the ethanol and methanol extracts of M. indica leaf possess antibacterial activities. Further molecular characterization techniques are required to classify and identify the bioactive compounds of M. indica for enhanced drug development.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML