Nana Yaw Asiedu1, Yaa Adoma Asiedu2, Phanuel Coffie Kofi Bediako3
1Department of chemical Engineering, College of Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
2School of Chemistry, University of Witwatersrand, Johannesburg, South Africa
3Formerly Department of Chemical Engineering, College of Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Correspondence to: Nana Yaw Asiedu, Department of chemical Engineering, College of Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Micro-organisms which are believed to metabolize waste petroleum oils from soaked soils in some areas in Kumasi metropolis-Ghana were studied. The studies investigate the isolation, characterization and mode of action of the organisms with petroleum contaminated soils using available equipment and information to their appropriate genera. The analysis classified the isolates into the aerobic and micro-aerophilic categories. Isolates metabolic activities on the various petroleum fractions (petrol, kerosene, engine oil, and diesel) gave the following percentages for profuse growth: 0.1-10%-petrel, 0.1-3.8%-kerosene, 0.1-2.3%-engine oil and 0.1-3.8%-diesel. Morphological characteristics of the isolates were identical to the average dimension of colonies ranging between 3.00mm and 4.90mm and cell sizes between 0.70mm and 1.60mm. The isolates showed gram-negative reactions. When the isolates were cultured for the same period and using the equal amounts of suspension, petrol gave 4.21 x 105 cells/ml suspension, engine oil gave 3.78 x 105 cells/ml suspension, kerosene gave 2.64 x 105 cells/ml suspension and diesel oil gave 1.50 x105 cells /ml suspension by total microbial count techniques. The isolates thus studied appear to belong to the following families: Pseudomonadaceae, Asotobacteraraceae, Methylomodaceae and Halobacteraceae.
Keywords:
Isolation, Characterization, Microorganisms, Waste Petroleum Oils
Cite this paper: Nana Yaw Asiedu, Yaa Adoma Asiedu, Phanuel Coffie Kofi Bediako, Isolation, Identification and Characterization of Some Petroleum Metabolizers from Soils Contaminated with Petroluem Oils in Kumasi Metropolis - Ghana, Journal of Microbiology Research, Vol. 4 No. 2, 2014, pp. 117-124. doi: 10.5923/j.microbiology.20140402.12.
1. Introduction
In a developing country like Ghana petroleum resources arise the key fuel for energy supply on which most of the possibilities for economic development depend to a large extent. It plays an essential part of the lives of people in Ghana. Petroleum products such as kerosene are used in many homes for cooking and lighting. In the rural areas for instance it is a very important essential commodity. Gasoline, or petrol, Engine oil and lube oils are also used in automobiles and industrial engines. Generally, the deployment of petroleum products in both domestic and industrial activities essential to the development of the country generates variety of wastes. The wastes have to be pretreated before disposal or recycled so that they do not adversely affect the environment. The chemical nature of these wastes is so diverse depending upon the type of industrial/domestic activity, and there is no single method of pretreatment that can be prescribed. Special methods have to be developed to treat petroleum waste from a particular type of industry. Waste petroleum engine oils are generated by automobiles and by almost every kind of industrial activity. Disposal of these has been the focus of attention in recent times. Petroleum pollution can be on a small scale, where the waste products are dumped into streams or are used to service vehicles by auto- mechanics. This latter type of pollution is a minor and occurs in most garages. Petroleum oil waste may pose serious problems to local fauna and flora,eg vegetation, fish and human lives. On a large scale, the following are some of the environmental disasters caused by oil pollution, [ref]• On 3rd June 1979, the worst oil spills occurred after a blow-out beneath the drilling rig Ixtoc I in the gulf of Campeche, Mexico. The slick reached 640km by 5th August 1979.• The worst coastal damage occurred in March 1989 when the Exxon Valdez oil tanker ran aground in Prince William Sound Alaska, USA and spilled more than 30 000 tonnes of oil. Over 240km of coast were polluted and the company was fined $5 billion on top of clean –up bill of $3 billion.• The worst land pollution occurred from February to October 1994, following a burst of a pipeline, thousand of tonnes of crude oil flowed across the Pristine Arctic Tundra in the Komi region, Russia. Estimates of oil lost ranges from 60 000 to 280 000 tonnes in a slick 18km long, Guinness Book of Knowledge [1].Whilst no record of any major oil disaster since Ghana became an oil producing country in recent times. The minor oil waste pollution of the local environment is worth noting. Extensive work has been done by Boakye and Kumar [2] on the major cities and towns in Ghana which including Accra, Kumasi, Tarkoradi, and the mining districts of Obuasi. The first four areas (Accra, Kumasi, Tarkoradi and Obuasi) were chosen because of their developed industrial base, good road and rail connections and their relative nearness to each other. A total of 184 sample sites were surveyed. The results showed that 4991 kiloliters of waste oils are generated per year in the areas surveyed. Out of this only 1788 kiloliters per year (36%) were reused and the rest 3203 kiloliters (64%) go to waste. Such quantities of waste petroleum oils must be properly treated and /or disposed off, since it is a threat to the environment. Zobel [3], reviewed the action of micro-organisms on hydrocarbons and recognized that many micro-organisms have the ability to utilize hydrocarbons as a sole source of energy and carbon and that such micro-organisms are widely distributed in nature, he further recognized that the microbial utilization of hydrocarbons was highly dependent on the chemical nature of the compounds within the petroleum mixture and on environmental determinants. Twenty-one year after Zobel’s [3] classic review,-super tanker, Torrey canyon sank in the English Channel and with this incidence attention of the scientific community was dramatically focused on the problems of oil pollution. Disposal techniques proposed to combat this oil pollution thus include the following:• Incineration:This is very easy to carry out but has serious environmental consequences. Commercial lubricating oils contain metal compounds such as lead tetra-ethyl as additives to improve upon performance of engines and harmful metallic oxides produced are released into the atmosphere with the combustion gases.• Treating with detergents:This is generally used for oil spills in the open seas. The detergents break the oil into smaller particles and disperse it through the water in micelles. These detergents are expensive and often non-biodegradable, thus adversely affect the environment. • Re-refining and recyclingThis seems to be the ideal way of petroleum waste disposal. This is an established industrial activity in several developed countries. It involves the treatment of sulphuric acid and activated clay and produces a waste acid clay sludge as a by product. This voluminous sludge itself poses a disposal problem and it is a major disadvantage of an otherwise simple technology. Newer reclaiming methods such as thin film vacuum distillation with hydro-finishing and solvent extraction are environmentally superior but require advanced technology and large capital investment, Mensah (1998).• Microbial methodsThe microbial treatment of waste petroleum oils for disposal is a new method in development that results in end-products that are completely friendly. Microbes disintegrate the hydrocarbon into smaller biodegradable products. These microbes feed on petroleum polluted areas and can be found in soils contaminated with petroleum dumps.Some of the organisms reported so far are strains of bacteria (Pseudomonas, Bacillus, and Methanomonas), actinomycetes and moulds. These organisms are not confined to one region. Wherever they are found to be associated with soils and other surfaces in which the petroleum fractions can be present may serve as a source of microbial inoccular, Beerstecher [4]. It is easy to look for these organisms in soils soaked with petroleum fractions. Thus if the soil serves as a substrate in which the organisms lives they can easily be isolated from the substrate and its characteristics determined. This work therefore aims at isolating, identifying and characterizing micro-organisms which may be present in soils contaminated with petroleum fractions, to study the actions of the isolates on various petroleum fractions and to optimize the parameters which influence their metabolic growth parameter, and design an appropriate bioreactor for further microbial studies of the isolates.
2. Materials and Methods
Survey:This work was carried out from September 1998 to April 1999 in the city Kumasi, a metropolis in the Ashanti Region of Ghana. Preliminary observations were done to determine the areas where samples could be taken. Based on the observation report four suburbs and sample locations in the city were chosen namely: Goil depot, Suame Magazine, Shell Ghana Limited depot, Kentenkrono car servicing and filling station. The contaminated soils found at these places were soaked with various petroleum products and so were ideal for isolation of petroleum metabolizing micro-organisms. Soil samples of soaked with different petroleum products (Petrol, Kerosene, Diesel, Engine oil) were taken from each survey area to determine the microbial load at each area and also isolate and identify in particular the different genera of bacteria present. They were obtained using intermittently sterilized hand shovel and placed in a transparent plastic polythene bags. About 20g of soil soaked with the product were randomly taken from the sites. Four samples were taken from each site.
3. Sterilization Procedure
Sterilization is the freeing of an article of all living organisms including bacteria and their pores. Various procedures are used to kill micro-organisms. The choice of method depends on the physical and chemical nature of the material to be sterilized, thus whether apparatus and materials are being prepared for use or for disposal and cleaning. Some of the methods used in this work include:• Autoclaving:Equipment like conical flasks, funnels and test-tubes were autoclaved at 15 Ib/in2 at 121 oC at 15 min. Distilled Water and media for bacterial growth were autoclaved at the same conditions of pressure, temperature and time to ensure total destruction of spores and also the biological media was autoclaved at the same conditions.• Hot-air oven operations:Petri-dishes, pipettes and other heat resistance glassware were wrapped in kitchen foil and were sterilized at 160 oC for about one hour in hot-air oven (Gallen Kamp OVH-520).• Flaming:Inoculating loops and wire, stab needles were sterilized by holding them in a Bunsen flame until they were red hot before and after used. The mouths of test –tubes, sample tubes, flasks, glass slides and pipettes were also decontaminated using such flames. • Ultra-violet light sterilization:Bacteria were killed in the inoculation chamber by radiations of ultra-violet light. The UV-lamp was switched on overnight to disinfect the upper air in the inoculation chamber. The light was switched off the following day for a period of at least one hour for the radiant energy to be absorbed into walls and floors before the chamber was used.• Chemical Sterilant:Quaternary ammonium compounds (quarts) are efficient both as micro- biostatic agents and detergent. Quarts such as teepol and omo (detergents) and distilled water were used to wash and rinse the equipment. 2% phenol which coagulates protein was used to inhibit and reduce microbial load after washing with omo. 70% ethyl alcohol which is bactericidal was used to swab the top of the working bench in the inoculating chamber before inoculations were performed.
4. Media
Culture media such as Nutrient Broth and Nutrient Agar and Plate count Agar were used in inducing growth of the micro-organisms and to determine viable counts of the micro-organisms. All media were dispensed into tubes and flasks sterilized at 121℃ for 15 minutes. Sterility test to determine the effectiveness of sterilization was done by keeping the media at room temperature for 72 hours. When microbial did not appear, such media was considered sterile, otherwise sterilization was not effective. The media used (Difco Products, Detroit, USA) had the following composition:• Peptone from meat• Meat Extract• Agar – Agar• Distilled waterThe preparation of the media followed the manufacturers’ instructions.
5. Isolation Procedure
• Sample Collection:Sampling took place in four areas in Kumasi. Table 1 below show sampling sites and soil samples soaked with various petroleum fractions:| Table 1. Sample sites and soils soaked with petroleum fraction sampled |
| | Sample Site | Petrol (P) | Kerosene (K) | Diesel (D) | Engine oil (EO) | | Shell Depot | | | | v | | Kentenkrono | | v | | v | | Suame Magazine | | | | v | | Goil Depot | v | v | v | v |
|
|
• Isolation of pure culturesØ Streak plate methodA sterile inoculating loop was dipped into the samples which was a mixed culture containing more than one type of microbes and is streaked over the surface of nutrient agar medium and incubated for 50 hours. Distinct isolated colonies were obtained and were picked up with another sterilized inoculating loop and transfer into sterilized test tubes containing nutrient broth medium to form pure Culture, Stainer et al [5], Collins [6]. Ø Pour plating methodSuccessive dilutions up to 10-6 of the inoculums were prepared and placed in sterile Petri dishes and mixed with cooled but molten nutrient agar medium and incubated for 50 hours. Distinct colonies were obtained inside and on the surface of the medium and they were used as pure cultures, Stainer et al (1992). • Maintenance of pure culturesPure cultures of the isolates were maintained on slants of nutrient agar and kept in a refrigerator at about 5℃. This was periodically sub-cultured for further studies.
6. Microbial Counts
• Plate countPlates showing about 30 – 200 colonies were selected from the incubator. Colonies on each selected plate were counted using the electronic colony counter. The total number if colonies were obtained by multiplying the average number of each plate by the reciprocal of the corresponding dilution.• Direct count –Estimated total bacterial countThe Helber counting chamber was used for the estimation. An etched, react angular slide of surface area of 100 mm2 was used. The surface of the slide was smeared with 0.01mL of bacterial suspension and went through the gram-staining techniques as described below. The microscope used was standardized by determining its objective lens diameter (D = 1.25 mm) and hence its surface area.The number of microscopic field (Mf) was calculated as follows: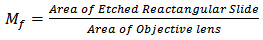 | (1) |
Random counts of cells from 10 fields were done and the average was determined. The total number of cells per 0.02mL was obtained by multiplying the microscopic field by the average number of cells obtained from the isolate.
7. Inoculation
In the inoculation chamber, platinum wire loop flamed red-hot was allowed to cool by touching the clear surface the medium before the culture to avoid killing of the micro-organisms. This was transferred onto the medium. Mouths of the test tubes were flamed before and after inoculation. It was ensured that test tubes and Petri dishes were opened too far from the flame during the time of inoculation.
8. Identification Procedure
In identifying the unknown spices of organisms, the culture provided broad information on the identity of the organisms. Gram staining techniques was employed to determine cell types of and the cell sizes of the bacteria. Colony characteristics were nearly specific for each microbial genus hence their importance in the identification processes. The following characteristics were taken into consideration: size of colony, elevation margin and color as seen on the agar.Stanier, et al [5], Breed, et al [7].• Gram staining techniqueThe method of Clifton [8] was followed in the Gram staining technique which is and identification requirement.The following steps were followed:§ Isolates were fixed on clean glass slides.§ 1% gentian violet was added for a minute and washed with distilled water.§ Gram iodine was then added for a minute and decolorized with 95% acetone-alcohol mixture and washed with distilled water.§ The isolates were then counter-stained with safranin solution for one minute and then washed with a slow jet of tap water.§ Slides were then blotted dry for microscopic studies.The staining made it possible to classify the micro-organisms into two broad classes of Bacillus and Cocci.
9. Results
9.1. Growth Trends of the Isolates in Various Petroleum Fractions
Preliminary growth studies undertaken using general bacteria medium comprising of nutrient broth and its agar with the following percentage of the petroleum fractions are shown in the figures below: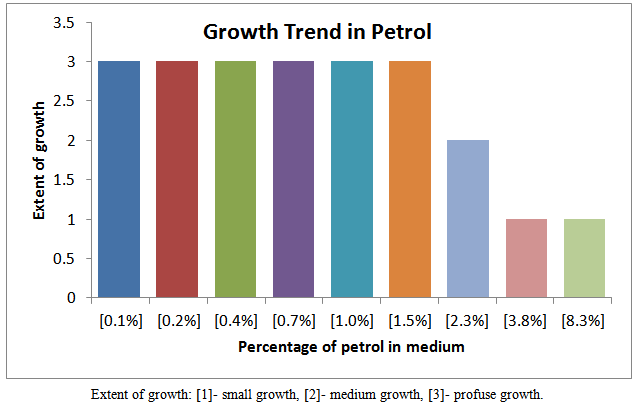 | Figure 1. Growth trend of isolates in various fraction of petro |
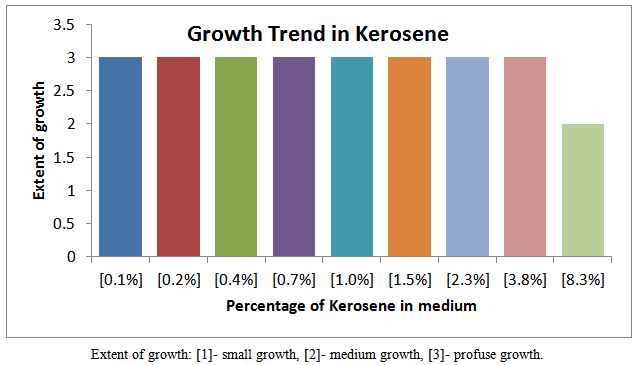 | Figure 2. Growth trend of isolates in various fraction of Kerosene |
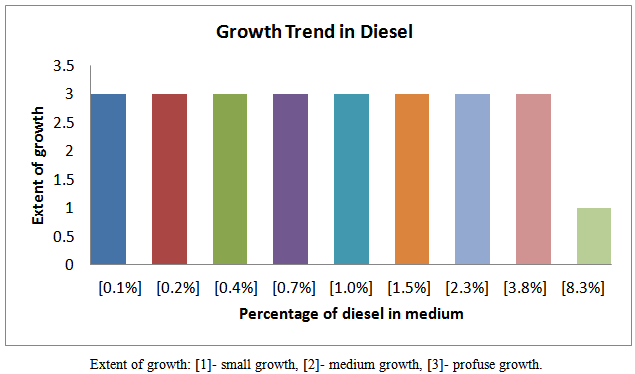 | Figure 3. Growth trend of isolates in various fraction of diesel |
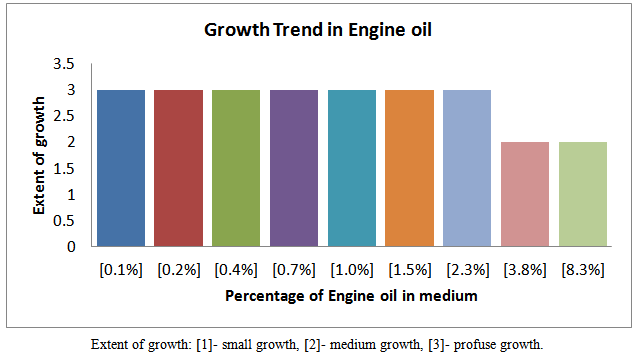 | Figure 4. Growth trend of isolates in various fraction of Engine oil |
9.2. Morphological Characteristics of the Microbial Isolates
Colony morphologies differ from bacteria from bacteria groups. In detail they also can help in identifying various genera of some bacteria. Table 2 below show a summary of the morphological characteristics of the isolates from the petroleum fractions understudy:| Table 2. Colony morphologies of the isolates from the petroleum fractions |
| | ISOLATES | COLONY MORPHOLOGY | OTHER CHARACTERISTICS | AVERAGE DIMENSION OF COLONY (mm) | | 0.2 % Petrol | Flat Circular, Irregular Translucent | Surface | 3.5 | | 0.4 % Petri | Flat Circular, Irregular Translucent | Surface | 3.5 | | 1.0 % Petrol | Flat Circular, Irregular Translucent | Surface | 3.7 | | 0.2 % Kerosene | Flat, Translucent | Surface/Body | 3.9 | | 0.4 % Kerosene | Rough/Flat, Translucent | Surface/Body | 4.3 | | 1.0 % Kerosene | Rough/Flat, Translucent | Surface/Body | 4.1 | | 0.2 % Diesel | Flat, Circular, Translucent | Surface | 3.9 | | 0.4 % Diesel | Flat, Circular, Translucent | Surface | 3.8 | | 1.0 % Diesel | Flat, Circular, Translucent | Surface | 3.7 | | 0.2 % Engine oil | Translucent, dotted and domed | Surface | 4.3 | | 0.4 % Engine oil | Translucent, dotted and domed | Surface | 4.9 | | 1.0 % Engine oil | Translucent, dotted and domed | Surface/Body | 4.9 |
|
|
9.3. Gram Reaction Characteristics of the Isolates
| Table 3. Characteristics of the isolates from the contaminated soils |
| | ISOLATES | GRAM REACTION | CELL SHAPE | CELL SIZE (μm) | GERMS NAME | | 0.2 % Petrol | - | Cocci in pairs/threes | 0.7 – 0.9 | Cocci | | 0.4 % Petri | - | Cocci in threes | 0.7 – 0.9 | Cocci | | 1.0 % Petrol | - | Cocci in pairs/threes | 0.7 – 0.9 | Cocci | | 0.2 % Kerosene | - | Short rods in pairs/threes | 0.9-1.4 | Bacillus | | 0.4 % Kerosene | - | Short rods in pairs/threes | 0.9-1.4 | Bacillus | | 1.0 % Kerosene | - | Short rods in pairs | 0.9-1.4 | Bacillus | | 0.2 % Diesel | - | Cocci in pairs, threes/ rods in pairs | 0.7-1.6 | Cocci /Bacillus | | 0.4 % Diesel | - | Cocci in pairs, threes/ rods in pairs | 0.7-1.5 | Cocci /Bacillus | | 1.0 % Diesel | ± | Cocci in pairs, threes/ rods in pairs | 0.7-1.0 | Cocci /Bacillus | | 0.2 % Engine oil | - | Cocci in pairs, threes | 0.7 – 0.9 | Cocci | | 0.4 % Engine oil | - | Cocci in pairs, threes | 0.7 – 1.0 | Cocci | | 1.0 % Engine oil | ± | Short rods in pairs/threes | 0.9- 1.4 | Bacillus |
|
|
9.4. Photomicrographs of the Isolates from the Various Fractions
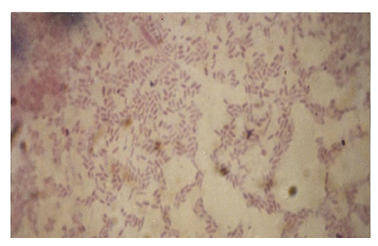 | Figure 7. Photomicrograph of isolates from Kerosene |
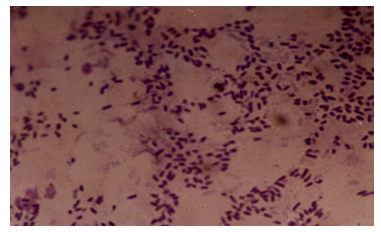 | Figure 8. Photomicrograph of isolates from Engine oil |
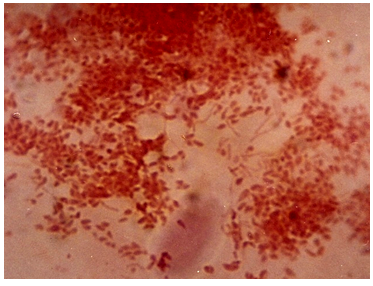 | Figure 9. Photomicrograph of isolates from Petrol |
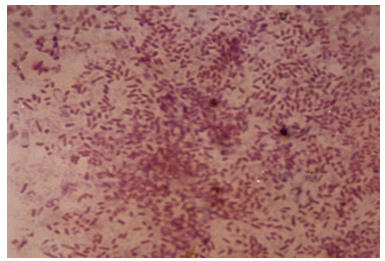 | Figure 10. Photomicrograph of isolates from Diesel |
10. Discussion of Results
Petroleum pollution problems are world–wide and affect animals, water-bodies and countries near-seashores. Several attempts have been made to address this problem of pollution. One of the recent methods is the application of micro-organisms which may be harmless to the environment and humans. It is therefore become necessary to isolate, identify and characterize the micro-organisms which could hydrolyze petroleum fractions such as petrol, kerosene, and diesel and engine oil. In this work the growth trend of cultures from soils soaked with the petroleum fractions as shown in figures 3, 4, 5 and 6 indicates that members of different families of micro-organisms survive in various fractions in nature. It is shown that these organisms survived in these fractions within 0.1 -2.3 % of the various fractions studied. It can be seen that growth trend appears to decrease in higher percentage of the fractions. This may be due to the nature of the final growth phase, where a decrease in living cells concentration occurs. This decline in growth trend may also be as a result of the toxic by-products, hash environment or depletion of nutrient supply. This work has shown that within 0.1 - 1.0 % of petrol some micro-organisms can be culture. Within 0.1 -3.8 %, 0.1 -3.8 %, and 0.1 -2.3 % kerosene, diesel and engine oil, respective, there is facilated profused growth of some petroleum metabolizers. The current study found petroleum product type-based colony morphologies of the isolates as shown in Table 2 above. Petrol and diesel isolates were flat, circular and translucent whiles isolate from kerosene and engine oil showed rough/flat translucent, dotted and domed. These morphologies are interesting in that they revealed the identities of different kinds of micro-organisms cultured. Another interesting finding was that isolates from petrol and diesel showed profuse surface growth. This indicates that the organisms are most likely to be aerobic micro-organisms. Engine oil isolate and diesel isolates showed growth in the body of the agar medium is an indication of presence of micro-aerophilic organisms. It is interesting to note that there were variations in average dimensions of the colonies, with petrol isolates having a minimum of 3.0 mm up to a maximum of 4.9 mm for engine oil isolates. This indicates that the growth rate or doubling times was higher in engine oil than petrol and kerosene. From the Gram stain reactions, characteristics of the nature of the cell walls of the isolates were revealed. This is also and identification procedure. From Table 3 it was revealed that isolates from all fractions showed Gram negative characteristics, this means that the isolates posses’ cell walls which consist of thin peptidoglycan layer. Some engine oil isolates showed Gram positive characteristics. The cell shapes observed during the Gram reaction test showed that cell types ranges from Cocci in pairs and in threes, short rods in pairs and in chains. The size ranges from 0.7μm to 1.6μm. From the Gram stain reaction, it became evidently clear that the isolates from the fractions could be grouped under the following bacteria: Tetrad, Gram negative mini-rods scattered and diplococcic. The Gram negative mini-rods although not worked out to genus level could belong to pseudomonadaceae. A typical morphological bacterium which was found by its Gram reaction was most likely to be a member of Azotobactereaceae. Since the culture was stained and only one shape was observed a suspicion of the presence of the genus Rhizobuim could changed from its imolution form to a definite Gram negative shape. An algorithm which is known to metabolize methane is Methylomonas a member of the family Methylononadaceae. It is an air-borne micro-organism known to be associated with petroleum hydrolysis. The organisms which could metabolize Halogens are the Halobacteria. Even though specifically no indexing was done for halobacteria, it could be that this organism may be a member of the isolates from the above, which has been reported by earlier workers, as being present in soil environments which mat contain petroleum products. These findings whiles preliminary suggest that detailed d characterization should be done to species level so that the isolates could be pin-pinpointed to specific micro-organisms. This is an important issue for future work.
11. Conclusions
The purpose of the present study was to isolate, identify and characterize micro-organisms which may be in soils contaminated with petroleum fractions in some areas in Kumasi metropolis in Ghana. The study has revealed that different micro-organisms especially bacteria have been isolated using standard microbiological techniques and media (Nutrient Broth and its agar). The most obvious finding emerged from this study is that the isolates were mostly Gram negative mini-rods and Cocci which have been reported to be associated with petroleum microbial organisms. The research also showed that the micro - organisms isolated showed aerobic and micro - aerophillic characteristics. The finding serve as a basis for future studies in petroleum metabolizers in soils contaminated with petroleum fractions in Ghana. The Authors intend to further the research by conducting characterization test of the isolates to species level.
ACKNOWLEGEMENTS
We thank Mr. K.O. Nyako, formerly of Biological Science Department, Kwame Nkrumah University of Science and Technology, Kumasi –Ghana and his team of technicians for their assistance and expertise made available to as during this work. We also express our sincere appreciation for the Kwame Nkrumah University of Science and Technology, Kumasi –Ghana for financial support for this work.
References
| [1] | Guinness Book of Knowledge, Guinness Media Inc., 1997, 1998. |
| [2] | Boakye, K., Kumar, A. Assesmant of used petroleum oil generation and its utilization in Ghana. 1995. Unpublished Data. |
| [3] | ZoBell, C. E. 1946. US. Patent 2413,278. |
| [4] | Beerstecher, E. An introduction to Microbiological Petroleum Engineering, Elsevier Press, Inc.,: Houston New York, 1954. |
| [5] | Stanier, R. Y., Albert, E. A., Ingraham, J. L. General Microbiology, Macmillan Publishers Limited, 1992. |
| [6] | Collins, C. H. Laboratory techniques of seris microbiological methods” Butterworth Co. Publishers Limited, London 2nd Ed. 1967. |
| [7] | Breed, R. S., Murray, E. G. D., Bergey, D. H. Berger’s Manual of determinative Bacteriology, Williams and Willins Co. Baltimore. 1975. |
| [8] | Clifton, C. E. Introduction to the Bacteria, McGraw-Hill Book Company, Inc. USA, 2nd Ed.1958. |

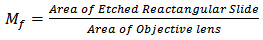








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML