-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(2): 86-91
doi:10.5923/j.microbiology.20140402.07
Antifungal Activities of Phyto Compounds from Mitracarpus villosus (Sw.) DC from Abuja, Nigeria
M. I. Aboh1, 2, B. O. Olayinka2, G. O. Adeshina2, P. Oladosu1
1Department of Microbiology, Human Virology and Biotechnology, National Institute for Pharmaceutical Research and Development, Idu. P.M.B 21 Garki, Abuja, Nigeria
2Department of Pharmaceutics and Pharmaceutical microbiology, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
Correspondence to: M. I. Aboh, Department of Microbiology, Human Virology and Biotechnology, National Institute for Pharmaceutical Research and Development, Idu. P.M.B 21 Garki, Abuja, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In recent years, there has been a gradual revival of interest in the use of medicinal plants in developing countries because herbal medicines have been reported to be well tolerated when compared with synthetic drugs. The powdered plant was screened for phyto-compounds. Crude tannins, phenols and saponins were extracted from the plant and quantified. Antifungal activities of the tannin, phenol and saponin extracts of Mitracarpus villosus against clinical isolates of Candida albicans, Candida krusei, Trichophyton verrucosum, Trichophyton mentagrophytes, Aspergillus fumigatus and Aspergillus niger were investigated using agar diffusion and micro broth dilution methods. The antibiotic susceptibility profiles of the fungal isolates to fluconazole and ketoconazole were also determined. Phytochemical screening revealed the presence of tannins, saponins, flavonoids, terpenes, phenols and resins. The percentage yield of the crude tannin, saponin and phenols were 1.4%, 17.0% and 9.0% respectively The diameter zones of inhibition at an exposure concentration of 12.5 mg/ml showed that the crude tannin extract produced the strongest antifungal activity against the yeasts with diameter zones of inhibition ranging from 21.67 – 23.67 mm while the crude saponin extract exhibited the strongest antifungal activity against the moulds with diameter zones of inhibition ranging from 21.00 – 24.67 mm. The minimum inhibitory concentration values of the crude tannin, phenol and saponin extracts against all the fungal isolates tested were 0.5 - 2.0, 0.5-8.0 and 0.5-4.0mg/ml respectively while the minimum fungicidal concentration values were 1.0 - 8.0, 1.0 - 16.0 and 1.0 - 8.0 mg/ml respectively. The plant has good potentials for development of new antifungal drugs.
Keywords: Antifungal, Phyto-compounds, Susceptibility, Minimum inhibitory concentration
Cite this paper: M. I. Aboh, B. O. Olayinka, G. O. Adeshina, P. Oladosu, Antifungal Activities of Phyto Compounds from Mitracarpus villosus (Sw.) DC from Abuja, Nigeria, Journal of Microbiology Research, Vol. 4 No. 2, 2014, pp. 86-91. doi: 10.5923/j.microbiology.20140402.07.
Article Outline
1. Introduction
- Plants have provided a source of inspiration for novel drug compounds, as plant derived medicines have made large contributions to human health and well-being [1]. Early humans recognized their dependence in nature in both health and illness. Led by instinct test and experience, primitive people treated illness by using plant, animal parts and minerals that were not part of their diet [2]. The medicinal value of plants is related in their phytochemical components which produce definite physiological actions on human body. The most important of these components are alkaloids, tannins, flavonoids and phenolic compounds [3]. Phytochemical compounds isolated from plants have been reported to possess anti fungal properties [4, 5].The plant Mitracarpus villosus (S.W) D.C belongs to the family Rubiaceae. It grows as a weed on old and abandoned farmlands and is distributed widely from forest to savanna zones of the tropics. In various parts of tropical Africa, it is traditionally used for treatment of sore throat. It has also been used to treat ringworm and eczema, fresh cuts, wounds and ulcer. In Nigeria, the extracted juice from aerial parts is topically applied against skin diseases and on wounds. [6]. Internally it is used as an antidote to arrow poison, anti-diarrhea, and anti-dysentery [6]. The extract from the native plant of Mitracarpus villosus is used treat the infection known as Dermatophilus congolensis of Cattle [7]. A decoction of its aerial part have been reported to have significant hepatoprotective effect against induced liver injury, both in vivo or in vitro [8]. Hexane extract of the leaves was found to have a dose dependent anti-inflammatory activity. The methanol extract is active against a wide range of test microorganisms, most remarkably Pseudomonas aeruginosa [9]. The aim of this study was to evaluate the antifungal activities of phyto compounds from Mitracarpus villosus aerial parts.
2. Materials and Methods
2.1. Collection and Identification of Plant Materials
- The fresh aerial parts of M. villosus was collected from the National Institute for Pharmaceutical Research and Development (NIPRD) garden. The plant was identified and authenticated in the herbarium of the Department of Medicinal Plant Research and Traditional Medicine, NIPRD, Abuja, Nigeria. The sample of the plant was deposited in the herbarium for reference purpose with Voucher specimen No. NIPRD/ H/ 4208.
2.2. Preparation of Crude Plant Material
- M. villosus aerial parts were air dried at room temperature for 10 days. The completely dried aerial parts were crushed to coarse powder by grinding with wooden mortar and pestle.
2.3. Phytochemical Screening of M. villosus
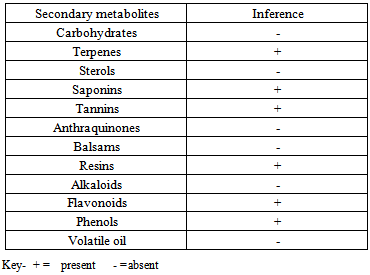
- The phytochemical screening for the presence or absence of secondary metabolites in M. villosus crude extracts of the fresh aerial part and dried aerial part was performed using standard methods. [10, 11, 12]. Test for CarbohydratesMolisch’s test: Few drops of Molisch's reagent was added to each of the portion dissolved in distilled water, this was then followed by addition of 1 ml of conc. H2SO4 by the side of the test tube. The mixture was then allowed to stand for two minutes and then diluted with 5 ml of distilled water. Formation of a red or dull violet colour at the interphase of the two layers was a positive test.Fehling’s test: About 0.5 g of the powdered plant was dissolved in distilled water and filtered. The filtrate was heated with 5 ml of equal volumes of Fehling's solution A and B. Formation of a red precipitate of cuprous oxide was an indication of the presence of reducing sugars.Test for tanninsa. Lead sub-acetate test: A few drops of lead sub-acetate were added to 1.0 ml of the powdered suspension. The colour change was noted.b. Bromine water test: Few drops of bromine water were added to 1.0 ml of the powdered plant suspension in a fume chamber. The colour change was observed.c. Ferric chloride test: Four milliliter of distilled water was added to 2.0 g of the powdered plant suspension. Few drops of Ferric chloride were added to the mixture. The colour reaction noted.d. Ferric ammonium chloride test: Ferric ammonium citrate solution (0.25%) was added to 1.0 ml of the powdered plant. Sufficient solid sodium acetate was added to the mixture to adjust the solution to pH 8 using pH indicator paper. This was boiled on a water bath at 80℃ and filtered. The colour reaction noted.Test for flavonoidsa. Magnesium chips or Shinoda test: The powdered plant (0.5 gm) was extracted in ethanol by boiling on a water bath for 5 min, filtered and cooled. A small quantity of Magnesium chips was added to the filtrate and few drops of concentrated HCl were added down the side of the test-tube. The colour change was noted.b. Zinc chips test: A small quantity of zinc chips was added to the powdered plant and few drops of concentrated HCl were added down the side of the test tube. The colour change was noted.Test for resinsa. Fifteen milliliters of plant were prepared using 0.1 gm of the powdered plant and filtered into a test-tube. An equal volume of copper acetate solution was added and shaken vigorously then allowed to separate. The colour change was noted.b. The powdered plant (0.5g) was dissolved in acetic anhydride and one drop of concentrated sulphuric acid was added. The colour change was noted.Test for SterolsLiebermann–Burchard test: Powdered plant suspension (1ml) was treated with chloroform, acetic anhydride and drops of H2SO4 was added and observed for the formation of dark pink or red colour.H2SO4 test: A fraction of plant was treated with ethanol and H2SO4 and observed for the formation of violet blue or green colour.Test for PhenolsFerric chloride test: A fraction of plant was treated with 5 % ferric chloride and observed for formation of deep blue or black colour.Liebermann’s test: The plant was heated with sodium nitrite, added H2SO4 solution diluted with water and excess of dilute NaOH was added and observed for the formation of deep red or green or blue colour.Test for AnthraquinonesBorntrager’s test: About 50 mg of powdered extract was heated with 10% ferric chloride solution and 1ml concentrated HCl. The extract was cooled, filtered and the filtrate was shaken with diethyl ether. The ether extract was further extracted with strong ammonia; pink or deep red colourations of aqueous layer indicate the presence of anthraquinone.Test for AlkaloidsWagner’s test: A fraction of the plant was treated with Wagner’s test reagent [1.27 g of iodine and 2 g of potassium iodide in 100 ml of water] and observed for the formation of reddish brown colour precipitate.Test for terpenes (Salkowski test): Five ml of each plant mixture was mixed in 2 ml of chloroform, and concentrated H2SO4 (3 ml) was carefully added to form a layer. A reddish brown colouration of the inter face was formed to show positive results for the presence of terpenoids.Test for saponin: About 2 g of the powdered sample was boiled (80-100℃) in 20 ml of distilled water in a water bath and filtered. 10ml of the filtrate was mixed with 5 ml of distilled water and shaken vigorously for a stable persistent froth. The frothing was mixed with 3 drops of olive oil and shaken vigorously, then observed for the formation of emulsion.
2.4. Extraction of Secondary Metabolites
2.4.1. Extraction of Crude Tannins
- One hundred milliliters of distilled water was added to 20.0g of powdered plant. This was allowed to boil for a minute and then filtered. The filtrate was mixed with 100.0 mL of ethyl acetate in a separating funnel. The ethyl acetate layer was evaporated to dryness and the residue was weighed [13].
2.4.2. Extraction of Saponins
- The sample (50 g) was extracted with 1 liter of aqueous methanol (50%) by cold extraction for 72 h. The extract was filtered using Whatman filter paper No. 2, and concentrated on a rotary evaporator (Buchi Rotavapor R-124) at 45ºC to give the crude methanol extract. The methanol crude was suspended in water saturated with n-butanol in a separating funnel. The n-butanol portion separated and collected from the aqueous portion. Diethyl ether (200 ml) was added to the n-butanol portion in a pre-weighed container, and crude saponin was precipitated [14].
2.4.3. Extraction of Phenols
- 200 g of the dried powdered plant was heated (80-100℃) with 1L of 2M Hydrochloric acid for 40 minutes in a water bath. The mixture was allowed to cool and the cold solution filtered. The phenol was extracted with diethyl ether three times and the combined extract evaporated to dryness and weighed [15].
2.5. Determination of Antifungal Activity of M. villosus
2.5.1. Cultivation and Standardization of Test Fungi
- Eighteen-hour broth culture of the test Candida spp. was suspended into sterile Sabouraud dextrose liquid medium. It was standardized according to Clinical Laboratory Standards Institute [16] by gradually adding normal saline to compare its turbidity to McFarland standard of 0.5 which is approximately 1.0×106 CFU/mL. However, for Trichophyton spp. and Aspergillus spp., fungal spores were harvested from 7 day old Sabouraud dextrose agar (SDA) slant cultures by washing with 10 mL sterile normal saline containing 3% w/v Tween 80 with aid of sterile glass beads to help in dispersing the spores [17]. Thereafter, the spore suspension was standardized to 1.0x106 spores/mL by using a single-beam spectrophotometer (Spectronic 20D; Milton Roy Company, Pacisa, Madrid, Spain) at 530 nm (OD530) of the suspensions and adjusted to 80 – 85% transmittance (Aspergillus spp.) and 70-72% transmittance (Trichophyton spp.). All adjusted suspensions were quantified by spreading 100μL on Sabouraud dextrose agar plate and incubated at 37°C for 18 h for yeast and 30°C for 72 hours for dermatophytes and moulds[18] (Aberkane et al., 2002). All cultures were checked for purity (by morphological growth on media, staining and biochemical tests) and maintained on Sabouraud dextrose agar (SDA) for the fungal test organisms respectively at 4ºC (in the refrigerator) until required for use.
2.5.2. Antifungal Screening of the Phyto Compounds of M. villosus
- Eighteen hours overnight cultures of Candida spp. and innoculum suspensions of the moulds and dermatophytes in Sabouraud dextrose liquid medium were standardized to produce inoculum size of 106 CFU/mL·1 mL of the diluted culture of each test organism was used to flood SDA plates and excess aseptically drained. The plates were allowed to dry at 37°C temperature in a sterilized incubator. Adopting the agar diffusion cup plate method [17], a sterile cork borer (6mm) was used to bore holes in the agar plates. The bottoms of the wells (holes) were sealed with the appropriate molten Sabouraud dextrose agar. Using micropipette, 0.1 mL each of the different graded concentrations of the ethyl acetate extract was dispensed into the holes. Distilled water and the solvents used in diluting the extracts were used as control. These were allowed to diffuse into the agar at room temperature for one hour before incubation at 37°C for 18 hours (yeast) and 30°C for 72 hours (dermatophytes and moulds). The zones of inhibition of the test organisms were measured to the nearest millimeter, using a well-calibrated meter ruler. The experiment was carried out in duplicates [19].
2.5.3. Determination of Minimum Inhibitory Concentration (M.I.C.) of the Phyto Compounds
- The M.I.C. of the phyto compounds and reference antibiotics to the test fungi was determined by serial broth microdilution method [20]. This assay was performed using round bottom 96-well clear microtitre plates. The cultured microplates were sealed with parafilm and incubated at 37°C for 18 hours (yeast) and 30°C for 48 hours (dermatophytes and moulds). M.I.C. was defined as the first well with no visible growth after incubation. The experiment was carried out in duplicates (each experiment was done in triplicates).
2.5.4. Determination of Minimum Fungicidal Concentration (MFC) of the Phyto Compounds
- 50 μL of the wells that did not show any visible growth after M.I.C. determination were inoculated in fresh wells containing Saboraud dextrose broth and incubated at 37°C for 18 hours (yeast) and 30°C for 48 hours (dermatophytes and moulds). Minimum fungicidal concentration was determined as the lowest concentration resulting in no growth on subculture [21].
2.5.5. Statistical Analysis
- Results obtained were expressed as mean±standard deviation and analysed for significance using one way ANOVA (Smith’s Statistical Package version 2.80) at p < 0.05.
3. Results
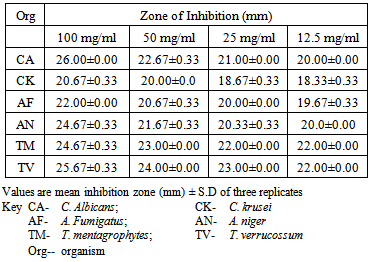
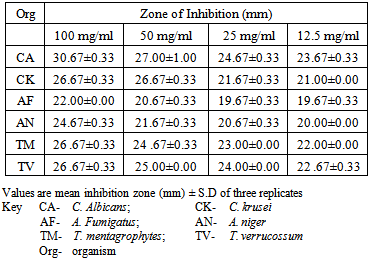
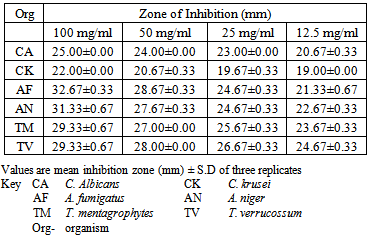
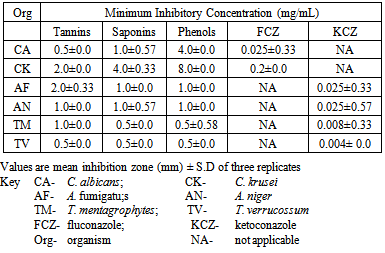
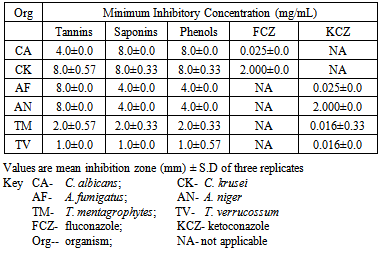
- The phytochemical screening of the powdered plant part revealed the presence of tannins, saponin, flavonoids, terpenes, phenols and resins while alkaloid, sugars, carbohydrates, balsams and anthraquinones were not detected (Table 1). The percentage yield of the crude tannin, saponin and phenols were 1.4%, 17.0% and 9.0% respectively The results of susceptibility testing are shown in Tables 2 – 4. Generally, an increase in the concentration of the phyto compounds led to an increase in antifungal activity shown with the increase in the diameter of zone of inhibition. The crude tannin extracts of M. villosus produced the strongest antifungal activity, followed by the crude saponin and finally the phenol extracts. The M.I.C. and M.F.C. values of the compounds on the fungal isolates are represented in Table 5 and 6.
|
|
|
|
|
|
4. Discussion
- All the secondary metabolites (phyto compounds) were active against the tested fungi. Phytochemical compounds isolated from plants have been reported to possess anti fungal properties [4, 5]. Presently, there is no documentation on the antifungal properties of secondary metabolites isolated from M. villosus however, studies by Irobi and Daramola (1993), revealed that the ethanol extracts of M. villosus was active against isolates of C. albicans and A. niger [22].In this study, the crude tannin from M. villosus had strong antifungal activities against all the fungi tested with anti fungal activities comparable with the standard drugs used. Increase in concentration of the extract led to a significant increase in antifungal activites (P < 0.05). It has been reported that tannins act as an antifungal agent at higher concentrations by coagulating the protoplasm of the micro-organism [23]. Similarly, the possible mechanism of action of tannins have been linked to interference with energy generation by uncoupling oxidative phosphorylation or interference with glycoprotein of cell surface [24].Saponins are naturally occurring surface-active glycosides. They have been reported to possess strong antifungal activities [4]. The major mechanism suggested for the antifungal activity of saponins is their interaction with membrane sterols. The antifungal activities of the crude saponin from M. villosus against the test fungi were comparable with the standard drugs. Increase in the concentration led to a significant increase in the antifungal activities (P < 0.05). The crude saponin at a concentration of 12.5 mg/mL exhibited good antifungal activity as indicated by high zones of inhibition against the yeast, moulds and dermatophytes, which was comparable with fluconazole and ketoconazole at a concentration of 0.05 mg/mL. Natural phenolic compounds are widespread in the plant kingdom. They are found in leaves, fruits, barks and wood and can accumulate in large amounts in particular organs or tissues of the plant [25]. The result of this study revealed that the phenol extracts of M. villosus produced the least antifungal activity against the test fungi than the tannin and saponin extracts of M. villosus, However increase in concentration led to significant increase in activity (P < 0.05). The mode of antifungal action of phenolic extract might be related to their ability to inactive adhesions, enzymes, cell envelope transport proteins [26] or related to the sites and number of OH group on the phenolic rings [27].
5. Conclusions
- In conclusion, the crude tannins, saponins and phenolic compounds showed good antifungal activity. The results confirm the claims of traditional healers in the use of the plant. This plant holds great promise for development into phytomedicine for the treatment of fungal infections in the near future.
ACKNOWLEDGEMENTS
- Our sincere appreciation goes to The DG/CEO of the National Institute for Pharmaceutical Research and Development (NIPRD) Prof. K. S. Gamaniel, Prof. Ibrahim Kolo for his assistance and technologists in the Bacteriology Unit of the Department of Microbiology and Biotechnology, NIPRD, Abuja.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML